Immune Netw.
2010 Dec;10(6):219-229. 10.4110/in.2010.10.6.219.
NDRG2-mediated Modulation of SOCS3 and STAT3 Activity Inhibits IL-10 Production
- Affiliations
-
- 1Department of Biological Science and the Research Center for Women's Diseases, Sookmyung Women's University, Seoul 140-742, Korea. jslim@sookmyung.ac.kr
- 2Department of Molecular Science and Technology, Ajou University, Suwon 443-749, Korea.
- KMID: 2150678
- DOI: http://doi.org/10.4110/in.2010.10.6.219
Abstract
- BACKGROUND
N-myc downstream regulated gene 2 (NDRG2) is a member of the NDRG gene family. Our previous report indicated a possible role for NDRG2 in regulating the cytokine, interleukin-10 (IL-10), which is an important immunosuppressive cytokine. Several pathways, including p38-MAPK, NF-kappaB, and JAK/STAT, are used for IL-10 production, and the JAK/STAT pathway can be inhibited in a negative feedback loop by the inducible protein, SOCS3. In the present study, we investigated the effect of NDRG2 gene expression on IL-10 signaling pathway that is modulated via SOCS3 and STAT3.
METHODS
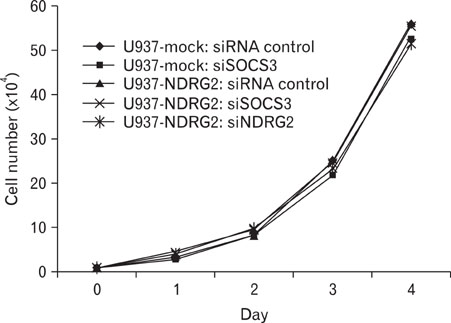
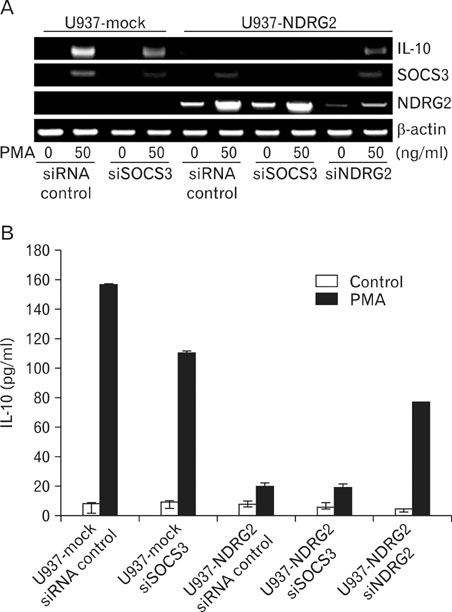
We generated NDRG2-overexpressing U937 cell line (U937-NDRG2) and treated the cells with PMA to investigate the role of NDRG2 in IL-10 production. U937 cells were also transfected with SOCS3- or NDRG2-specific siRNAs to examine whether the knockdown of SOCS3 or NDRG2 influenced IL-10 expression. Lastly, STAT3 and SOCS3 induction was measured to identify the signaling pathway that was associated with IL-10 production.
RESULTS
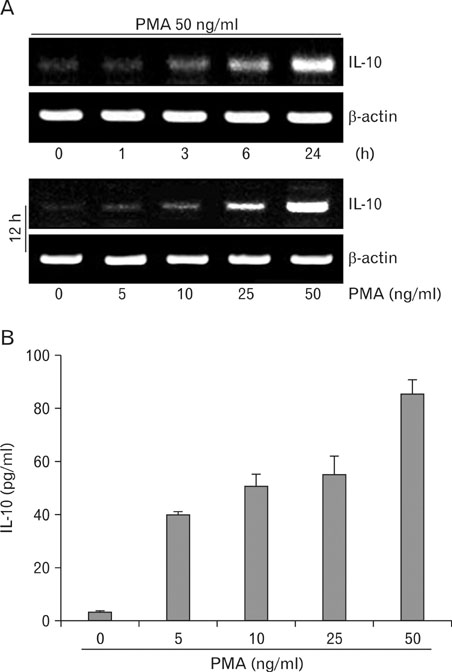
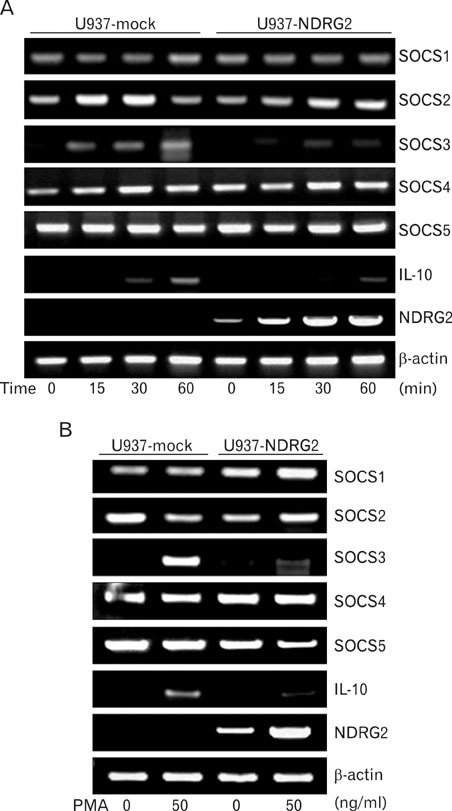
RT-PCR and ELISA assays showed that IL-10 was increased in U937-mock cells upon stimulation with PMA, but IL-10 was inhibited by overexpression NDRG2. After PMA treatment, STAT3 phosphorylation was decreased in a time-dependent manner in U937-mock cells, whereas it was maintained in U937-NDRG2 cells. SOCS3 was markedly reduced in U937-NDRG2 cells compared with U937-mock cells. IL-10 production after PMA stimulation was reduced in U937 cells when SOCS3 was inhibited, but this effect was less severe when NDRG2 was inhibited.
CONCLUSION
NDRG2 expression modulates SOCS3 and STAT3 activity, eventually leading to the inhibition of IL-10 production.
MeSH Terms
Figure
Cited by 1 articles
-
NDRG2 Promotes GATA-1 Expression through Regulation of the JAK2/STAT Pathway in PMA-stimulated U937 Cells
Kyeongah Kang, Hyeyoun Jung, Sorim Nam, Jong-Seok Lim
Immune Netw. 2011;11(6):348-357. doi: 10.4110/in.2011.11.6.348.
Reference
-
1. Kim YJ, Yoon SY, Kim JT, Choi SC, Lim JS, Kim JH, Song EY, Lee HG, Choi I, Kim JW. NDRG2 suppresses cell proliferation through down-regulation of AP-1 activity in human colon carcinoma cells. Int J Cancer. 2009. 124:7–15.
Article2. Choi SC, Kim KD, Kim JT, Kim JW, Yoon DY, Choe YK, Chang YS, Paik SG, Lim JS. Expression and regulation of NDRG2 (N-myc downstream regulated gene 2) during the differentiation of dendritic cells. FEBS Lett. 2003. 553:413–418.
Article3. Park Y, Shon SK, Kim A, Kim KI, Yang Y, Cho DH, Lee MS, Lim JS. SOCS1 induced by NDRG2 expression negatively regulates STAT3 activation in breast cancer cells. Biochem Biophys Res Commun. 2007. 363:361–367.
Article4. Liu N, Wang L, Li X, Yang Q, Liu X, Zhang J, Zhang J, Wu Y, Ji S, Zhang Y, Yang A, Han H, Yao L. N-Myc downstream-regulated gene 2 is involved in p53-mediated apoptosis. Nucleic Acids Res. 2008. 36:5335–5349.
Article5. O'Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev. 2008. 223:114–131.6. Choi YK, Kim YJ, Park YH, Choi KS, Park JG. Effective inhibition of glomerulosclerosis by adenoviral vector expressing human IL-10. Korean J Immunol. 2000. 22:187–195.7. Staples KJ, Smallie T, Williams LM, Foey A, Burke B, Foxwell BM, Ziegler-Heitbrock L. IL-10 induces IL-10 in primary human monocyte-derived macrophages via the transcription factor Stat3. J Immunol. 2007. 178:4779–4785.
Article8. Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998. 161:3299–3306.9. Dai WJ, Köhler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997. 158:2259–2267.10. Weber-Nordt RM, Riley JK, Greenlund AC, Moore KW, Darnell JE, Schreiber RD. Stat3 recruitment by two distinct ligand-induced, tyrosine-phosphorylated docking sites in the interleukin-10 receptor intracellular domain. J Biol Chem. 1996. 271:27954–27961.
Article11. Wehinger J, Gouilleux F, Groner B, Finke J, Mertelsmann R, Weber-Nordt RM. IL-10 induces DNA binding activity of three STAT proteins (Stat1, Stat3, and Stat5) and their distinct combinatorial assembly in the promoters of selected genes. FEBS Lett. 1996. 394:365–370.
Article12. Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol. 1995. 155:1079–1090.13. O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004. 10:801–805.14. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004. 4:499–511.
Article15. Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010. 10:170–181.
Article16. Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004. 172:567–576.
Article17. Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007. 8:1363–1371.
Article18. Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008. 180:2752–2756.
Article19. Xu J, Yang Y, Qiu G, Lal G, Wu Z, Levy DE, Ochando JC, Bromberg JS, Ding Y. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol. 2009. 182:6226–6236.
Article20. Kalliolias GD, Ivashkiv LB. IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J Immunol. 2008. 180:6325–6333.
Article21. Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006. 17:173–188.
Article22. Qin H, Niyongere SA, Lee SJ, Baker BJ, Benveniste EN. Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J Immunol. 2008. 181:3167–3176.
Article23. Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004. 22:503–529.
Article24. Yoshimura A, Nishinakamura H, Matsumura Y, Hanada T. Negative regulation of cytokine signaling and immune responses by SOCS proteins. Arthritis Res Ther. 2005. 7:100–110.25. Ding Y, Chen D, Tarcsafalvi A, Su R, Qin L, Bromberg JS. Suppressor of cytokine signaling 1 inhibits IL-10-mediated immune responses. J Immunol. 2003. 170:1383–1391.
Article26. Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, Hirano T, Chien KR, Yoshimura A. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003. 4:551–556.
Article27. Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol. 2002. 2:410–416.
Article28. Murray PJ. STAT3-mediated anti-inflammatory signalling. Biochem Soc Trans. 2006. 34:1028–1031.
Article29. Zhou RH, Kokame K, Tsukamoto Y, Yutani C, Kato H, Miyata T. Characterization of the human NDRG gene family: a newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics. 2001. 73:86–97.
Article30. Choi SC, Kim KD, Kim JT, Kim JW, Lee HG, Kim JM, Jang YS, Yoon DY, Kim KI, Yang Y, Cho DH, Lim JS. Expression of human NDRG2 by myeloid dendritic cells inhibits down-regulation of activated leukocyte cell adhesion molecule (ALCAM) and contributes to maintenance of T cell stimulatory activity. J Leukoc Biol. 2008. 83:89–98.
Article31. Bradshaw CD, Ella KM, Qi C, Sansbury HM, Wisehart-Johnson AE, Meier KE. Effects of phorbol ester on phospholipase D and mitogen-activated protein kinase activities in T-lymphocyte cell lines. Immunol Lett. 1996. 53:69–76.
Article32. Schultz H, Engel K, Gaestel M. PMA-induced activation of the p42/44ERK- and p38RK-MAP kinase cascades in HL-60 cells is PKC dependent but not essential for differentiation to the macrophage-like phenotype. J Cell Physiol. 1997. 173:310–318.
Article33. Sengupta TK, Talbot ES, Scherle PA, Ivashkiv LB. Rapid inhibition of interleukin-6 signaling and Stat3 activation mediated by mitogen-activated protein kinases. Proc Natl Acad Sci U S A. 1998. 95:11107–11112.
Article34. Terstegen L, Gatsios P, Bode JG, Schaper F, Heinrich PC, Graeve L. The inhibition of interleukin-6-dependent STAT activation by mitogen-activated protein kinases depends on tyrosine 759 in the cytoplasmic tail of glycoprotein 130. J Biol Chem. 2000. 275:18810–18817.
Article35. Terstegen L, Maassen BG, Radtke S, Behrmann I, Schaper F, Heinrich PC, Graeve L, Gatsios P. Differential inhibition of IL-6-type cytokine-induced STAT activation by PMA. FEBS Lett. 2000. 478:100–104.
Article36. Lagunas L, Clipstone NA. Deregulated NFATc1 activity transforms murine fibroblasts via an autocrine growth factor-mediated Stat3-dependent pathway. J Cell Biochem. 2009. 108:237–248.
Article37. Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004. 10:48–54.
Article38. Borland G, Bird RJ, Palmer TM, Yarwood SJ. Activation of protein kinase Calpha by EPAC1 is required for the ERK- and CCAAT/enhancer-binding protein beta-dependent induction of the SOCS-3 gene by cyclic AMP in COS1 cells. J Biol Chem. 2009. 284:17391–17403.
Article39. Boyle K, Robb L. The role of SOCS3 in modulating leukaemia inhibitory factor signalling during murine placental development. J Reprod Immunol. 2008. 77:1–6.
Article40. Fukushima A, Kajiya H, Izumi T, Shigeyama C, Okabe K, Anan H. Pro-inflammatory cytokines induce suppressor of cytokine signaling-3 in human periodontal ligament cells. J Endod. 2010. 36:1004–1008.
Article41. Dong Q, Fan R, Zhao S, Wang Y. Over-expression of SOCS-3 gene promotes IL-10 production by JEG-3 trophoblast cells. Placenta. 2009. 30:11–14.
Article42. Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, Bazzoni F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J Immunol. 2002. 168:6404–6411.
Article43. Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, Heinrich PC, Müller-Newen G. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003. 170:3263–3272.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- SOCS3 Attenuates DexamethasoneInduced M2 Polarization by DownRegulation of GILZ via ROS- and p38 MAPK-Dependent Pathways
- Sinensetin Inhibits Interleukin-6 in Human Mast Cell - 1 Via Signal Transducers and Activators of the Transcription 3 (STAT3) and Nuclear Factor Kappa B (NF-κB) Pathways
- Resveratrol Inhibits IL-6-Induced Transcriptional Activity of AR and STAT3 in Human Prostate Cancer LNCaP-FGC Cells
- Glutamine Deprivation Causes Hydrogen Peroxide-induced Interleukin-8 Expression via Jak1/Stat3 Activation in Gastric Epithelial AGS Cells
- IL-33 promotes IL-10 production in macrophages: a role for IL-33 in macrophage foam cell formation