Electrolyte Blood Press.
2007 Jun;5(1):15-22. 10.5049/EBP.2007.5.1.15.
Regulation of AQP2 in Collecting Duct: An emphasis on the Effects of Angiotensin II or Aldosterone
- Affiliations
-
- 1Department of Biochemistry and Cell Biology, School of Medicine, Kyungpook National University, Daegu, Korea. thkwon@knu.ac.kr
- KMID: 2134800
- DOI: http://doi.org/10.5049/EBP.2007.5.1.15
Abstract
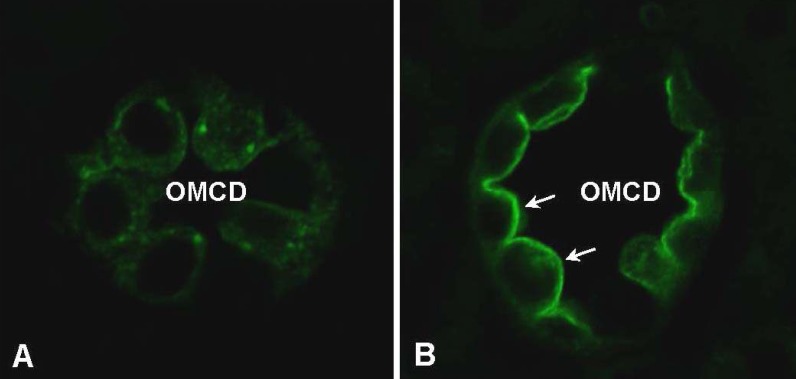
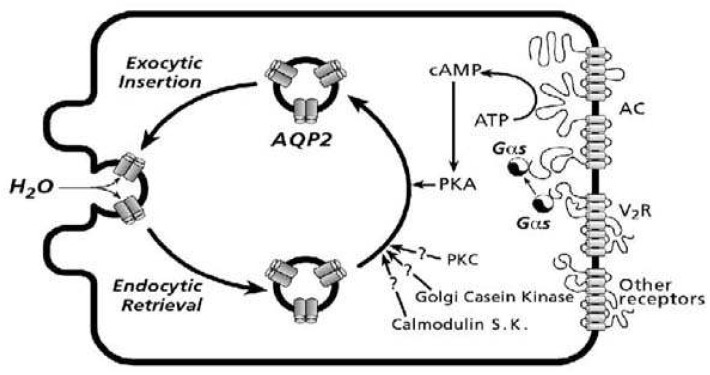
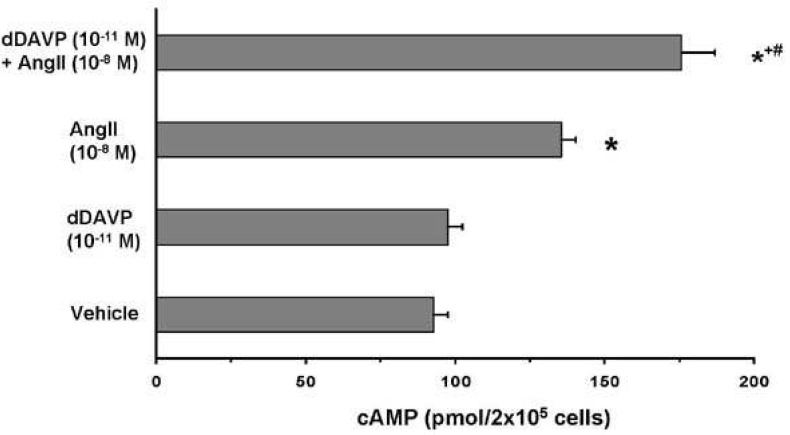
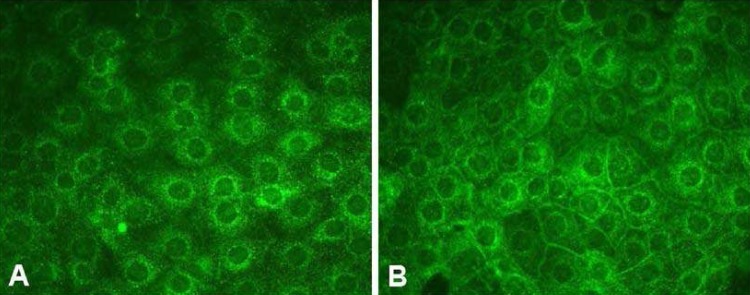
- Vasopressin, angiotensin II (AngII), and aldosterone are essential hormones in the regulation of body fluid homeostatsis. We examined the effects of AngII or aldosterone on the regulation of body water balance. We demonstrated that 1) short-term treatment with AngII in the primary cultured inner medullary collecting duct cells played a role in the regulation of AQP2 targeting to the plasma membrane through AT1 receptor activation. This potentiated the effects of dDAVP on cAMP accumulation, AQP2 phosphorylation, and AQP2 plasma membrane targeting; 2) pharmacological blockade of the AngII AT1 receptor in rats co-treated with dDAVP and dietary NaCl-restriction (to induce high plasma endogenous AngII) resulted in an increase in urine production, a decrease in urine osmolality, and blunted the dDAVP-induced upregulation of AQP2; 3) long-term aldosterone infusion in normal rats or in rats with diabetes insipidus was associated with polyuria and decreased urine concentration, accompanied by decreased apical but increased basolateral AQP2 labeling intensity in the connecting tubule and cortical collecting duct; and 4) in contrast to the effects of dDAVP and AngII, short-term aldosterone treatment does not alter the intracellular distribution of AQP2. In conclusion, angiotensin II, and aldosterone could play a role in the regulation of renal water reabsorption by changing intracellular AQP2 targeting and/or AQP2 abundance, in addition to the vasopressin.
MeSH Terms
-
Aldosterone*
Angiotensin II*
Angiotensins*
Animals
Aquaporin 2
Body Fluids
Body Water
Cell Membrane
Deamino Arginine Vasopressin
Diabetes Insipidus
Osmolar Concentration
Phosphorylation
Plasma
Polyuria
Rats
Up-Regulation
Vasopressins
Water-Electrolyte Balance
Aldosterone
Angiotensin II
Angiotensins
Aquaporin 2
Deamino Arginine Vasopressin
Vasopressins
Water-Electrolyte Balance
Figure
Reference
-
1. Knepper MA, Kim GH, Fernandez-Llama P, Ecelbarger CA. Regulation of thick ascending limb transport by vasopressin. J Am Soc Nephrol. 1999; 10:628–634. PMID: 10073614.
Article2. Knepper MA, Nielsen S, Chou CL, DiGiovanni SR. Mechanism of vasopressin action in the renal collecting duct. Semin Nephrol. 1994; 14:302–321. PMID: 7938946.3. Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. Aquaporin water channels - from atomic structure to clinical medicine. J Physiol. 2002; 542:3–16. PMID: 12096044.
Article4. Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney : from molecules to medicine. Physiol Rev. 2002; 82:205–244. PMID: 11773613.
Article5. Kwon TH, Hager H, Nejsum LN, Andersen ML, Frokiaer J, Nielsen S. Physiology and pathophysiology of renal aquaporins. Semin Nephrol. 2001; 21:231–238. PMID: 11320486.
Article6. Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993; 361:549–552. PMID: 8429910.
Article7. Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem. 1997; 272:14800–14804. PMID: 9169447.
Article8. Christensen BM, Zelenina M, Aperia A, Nielsen S. Localization and regulation of PKA-phosphorylated AQP2 in response to V(2)-receptor agonist/antagonist treatment. Am J Physiol Renal Physiol. 2000; 278:F29–F42. PMID: 10644653.9. Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A. 1995; 92:1013–1017. PMID: 7532304.
Article10. Harris PJ, Navar LG. Tubular transport responses to angiotensin. Am J Physiol. 1985; 248:F621–F630. PMID: 3887946.
Article11. Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002; 13:1131–1135. PMID: 11960999.12. Wong NL, Tsui JK. Angiotensin II upregulates the expression of vasopressin V2 mRNA in the inner medullary collecting duct of the rat. Metabolism. 2003; 52:290–295. PMID: 12647265.
Article13. Kato A, Klein JD, Zhang C, Sands JM. Angiotensin II increases vasopressin-stimulated facilitated urea permeability in rat terminal IMCDs. Am J Physiol Renal Physiol. 2000; 279:F835–F840. PMID: 11053043.
Article14. Kwon TH, Nielsen J, Kim YH, Knepper MA, Frokiaer J, Nielsen S. Regulation of sodium transporters in the thick ascending limb of rat kidney : response to angiotensin II. Am J Physiol Renal Physiol. 2003; 285:F152–F165. PMID: 12657563.15. Kwon TH, Nielsen J, Knepper MA, Frokiaer J, Nielsen S. Angiotensin II AT1 receptor blockade decreases vasopressin-induced water reabsorption and AQP2 levels in NaCl-restricted rats. Am J Physiol Renal Physiol. 2005; 288:F673–F684. PMID: 15585668.16. Lee YJ, Song IK, Jang KJ, Nielsen J, Frokiaer J, Nielsen S, Kwon TH. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin II through AT1 receptor. Am J Physiol Renal Physiol. 2007; 292:F340–F350. PMID: 16896188.17. Edwards RM, Jackson BA, Dousa TP. ADH-sensitive cAMP system in papillary collecting duct: effect of osmolality and PGE2. Am J Physiol. 1981; 240:F311–F318. PMID: 6261588.
Article18. Karim Z, Defontaine N, Paillard M, Poggioli J. Protein kinase C isoforms in rat kidney proximal tubule: acute effect of angiotensin II. Am J Physiol. 1995; 269:C134–C140. PMID: 7631740.
Article19. Klingler C, Ancellin N, Barrault MB, Morel A, Buhler JM, Elalouf JM, Clauser E, Lugnier C, Corman B. Angiotensin II potentiates vasopressin-dependent cAMP accumulation in CHO transfected cells. Mechanisms of cross-talk between AT1A and V2 receptors. Cell Signal. 1998; 10:65–74. PMID: 9502119.
Article20. Hus-Citharel A, Marchetti J, Corvol P, Llorens-Cortes C. Potentiation of [Ca2+]i response to angiotensin III by cAMP in cortical thick ascending limb. Kidney Int. 2002; 61:1996–2005. PMID: 12028440.21. Star RA, Nonoguchi H, Balaban R, Knepper MA. Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct. J Clin Invest. 1988; 81:1879–1888. PMID: 2838523.
Article22. Nielsen J, Kwon TH, Praetorius J, Frokiaer J, Knepper MA, Nielsen S. Aldosterone increases urine production and decreases apical AQP2 expression in rats with diabetes insipidus. Am J Physiol Renal Physiol. 2006; 290:F438–F449. PMID: 16159898.
Article23. Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem. 2000; 275:36839–36846. PMID: 10973964.24. Katsura T, Verbavatz JM, Farinas J, Ma T, Ausiello DA, Verkman AS, Brown D. Constitutive and regulated membrane expression of aquaporin 1 and aquaporin 2 water channels in stably transfected LLC-PK1 epithelial cells. Proc Natl Acad Sci U S A. 1995; 92:7212–7216. PMID: 7543677.
Article25. Yamamoto T, Sasaki S, Fushimi K, Ishibashi K, Yaoita E, Kawasaki K, Marumo F, Kihara I. Vasopressin increases AQP-CD water channel in apical membrane of collecting duct cells in Brattleboro rats. Am J Physiol. 1995; 268:C1546–C1551. PMID: 7541941.
Article26. Yip KP. Coupling of vasopressin-induced intracellular Ca2+ mobilization and apical exocytosis in perfused rat kidney collecting duct. J Physiol. 2002; 538:891–899. PMID: 11826172.27. Lorenz D, Krylov A, Hahm D, Hagen V, Rosenthal W, Pohl P, Maric K. Cyclic AMP is sufficient for triggering the exocytic recruitment of aquaporin-2 in renal epithelial cells. EMBO Rep. 2003; 4:88–93. PMID: 12524527.
Article28. Bustamante M, Hasler U, Kotova O, Chibalin AV, Mordasini D, Rousselot M, Vandewalle A, Martin PY, Feraille E. Insulin potentiates AVP-induced AQP2 expression in cultured renal collecting duct principal cells. Am J Physiol Renal Physiol. 2005; 288:F334–F344. PMID: 15494547.
Article29. Kwon TH, Nielsen J, Masilamani S, Hager H, Knepper MA, Frokiaer J, Nielsen S. Regulation of collecting duct AQP3 expression: response to mineralocorticoid. Am J Physiol Renal Physiol. 2002; 283:F1403–F1421. PMID: 12388415.30. Nielsen J, Kwon TH, Frokiaer J, Knepper MA, Nielsen S. Lithium-induced NDI in rats is associated with loss of alpha-ENaC regulation by aldosterone in CCD. Am J Physiol Renal Physiol. 2006; 290:F1222–F1233. PMID: 16332930.31. Chen L, Williams SK, Schafer JA. Differences in synergistic actions of vasopressin and deoxycorticosterone in rat and rabbit CCD. Am J Physiol. 1990; 259:F147–F156. PMID: 2375388.
Article32. Handler JS, Preston AS, Orloff J. Effect of adrenal steroid hormones on the response of the toad's urinary bladder to vasopressin. J Clin Invest. 1969; 48:823–833. PMID: 5780194.
Article33. Green HH, Harrington AR, Valtin H. On the role of antidiuretic hormone in the inhibition of acute water diuresis in adrenal insufficiency and the effects of gluco- and mineralocorticoids in reversing the inhibition. J Clin Invest. 1970; 49:1724–1736. PMID: 5449709.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Minireview on Vasopressin-regulated Aquaporin-2 in Kidney Collecting Duct Cells
- Renal intercalated cells and blood pressure regulation
- Molecular analysis of AQP2 promoter. I. cAMP-dependent regulation of mouse AQP2 gene
- New insights into the transcriptional regulation of aquaporin-2 and the treatment of X-linked hereditary nephrogenic diabetes insipidus
- Recent Update of Renin-angiotensin-aldosterone System in the Pathogenesis of Hypertension