Tuberc Respir Dis.
2010 Oct;69(4):271-278. 10.4046/trd.2010.69.4.271.
PNA-Mediated PCR Clamping for the Detection of EGFR Mutations in Non-Small Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea. kyleemd@kuh.ac.kr
- 2Department of Pathology, Konkuk University School of Medicine, Seoul, Korea.
- KMID: 2114621
- DOI: http://doi.org/10.4046/trd.2010.69.4.271
Abstract
- BACKGROUND
Recent studies have demonstrated that the epidermal growth factor receptor (EGFR) genotype is the most important predictive marker to EGFR-tyrosine kinase inhibitors (TKIs) and first-line gefitinib treatment will be approved in the near future for use in non-small cell lung cancer (NSCLC) patients with the EGFR mutation. Direct sequencing is known to be the standard for detecting EGFR mutations; however, it has limited sensitivity. Peptide nucleic acids (PNA)-mediated PCR clamping method is a newly introduced method for analyzing EGFR mutations with increased sensitivity and stability.
METHODS
A total of 71 NSCLC patients were analyzed for EGFR mutations using the PNA-mediated PCR clamping technique. Sixty-nine patients were analyzed for clinicopathologic correlation with EGFR genotype; 2 patients with indeterminate results were excluded. In order to determine EGFR-TKI drug response, 57 patients (42 gefitinib, 15 erlotinib) were included in the analysis.
RESULTS
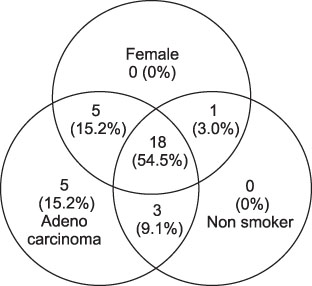
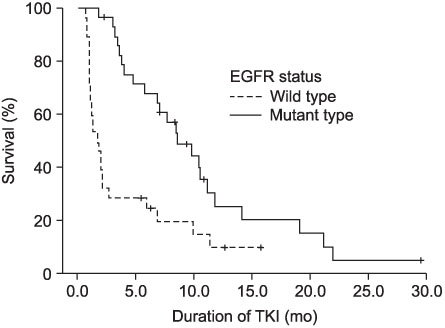
The EGFR mutation rate was 47.8%. Being female, a non-smoker, and having adenocarcinoma were favorable clinicopathologic factors, as expected. However, more than a few smokers (33.3%), male (28.1%), and patients with non-adenocarcinoma (28.6%) had the EGFR mutation. Having a combination of favorable clinicopathologic factors did not increase the EGFR mutation rate significantly. Drug response to EGFR-TKIs showed significant differences depending on the EGFR genotype; ORR was 14.3% for wild type vs 69.0% for mutant type; DCR is 28.6% for wild type vs 96.6% for mutant type. The median EGFR-TKI treatment duration is 7.6 months for mutant type group and 1.4 months for wild type group.
CONCLUSION
EGFR genotype determined using the PNA-mediated PCR clamping method is significantly correlated with the clinical EGFR-TKI responses and PNA-mediated PCR.
MeSH Terms
Figure
Cited by 1 articles
-
Peptide Nucleic Acid Clamping and Direct Sequencing in the Detection of Oncogenic Alterations in Lung Cancer: Systematic Review and Meta-Analysis
Jae-Uk Song, Jonghoo Lee
Yonsei Med J. 2018;59(2):211-218. doi: 10.3349/ymj.2018.59.2.211.
Reference
-
1. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004. 350:2129–2139.2. Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004. 304:1497–1500.3. Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol. 2005. 23:2556–2568.4. Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005. 97:643–655.5. Zhu CQ, da Cunha Santos G, Ding K, Sakurada A, Cutz JC, Liu N, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008. 26:4268–4275.6. Dacic S. EGFR assays in lung cancer. Adv Anat Pathol. 2008. 15:241–247.7. Pao W, Ladanyi M. Epidermal growth factor receptor mutation testing in lung cancer: searching for the ideal method. Clin Cancer Res. 2007. 13:4954–4955.8. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009. 361:947–957.9. Lee KY. Molecular diagnosis in lung cancer. J Lung Cancer. 2010. 9:9–14.10. Tanaka T, Nagai Y, Miyazawa H, Koyama N, Matsuoka S, Sutani A, et al. Reliability of the peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp-based test for epidermal growth factor receptor mutations integrated into the clinical practice for non-small cell lung cancers. Cancer Sci. 2007. 98:246–252.11. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010. 11:121–128.12. Hwang TS. Molecular biologic techniques in cytopathologic diagnosis. Korean J Pathol. 2009. 43:387–392.13. Kim SK, Kim DL, Han HS, Kim WS, Kim SJ, Moon WJ, et al. Pyrosequencing analysis for detection of a BRAFV600E mutation in an FNAB specimen of thyroid nodules. Diagn Mol Pathol. 2008. 17:118–125.14. Nagai Y, Miyazawa H, Huqun , Tanaka T, Udagawa K, Kato M, et al. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res. 2005. 65:7276–7282.15. Miyazawa H, Tanaka T, Nagai Y, Matsuoka M, Sutani A, Udagawa K, et al. Peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp-based detection test for gefitinib-refractory T790M epidermal growth factor receptor mutation. Cancer Sci. 2008. 99:595–600.16. Tanaka T, Matsuoka M, Sutani A, Gemma A, Maemondo M, Inoue A, et al. Frequency of and variables associated with the EGFR mutation and its subtypes. Int J Cancer. 2010. 126:651–655.17. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007. 7:169–181.18. Chou TY, Chiu CH, Li LH, Hsiao CY, Tzen CY, Chang KT, et al. Mutation in the tyrosine kinase domain of epidermal growth factor receptor is a predictive and prognostic factor for gefitinib treatment in patients with non-small cell lung cancer. Clin Cancer Res. 2005. 11:3750–3757.19. Cortes-Funes H, Gomez C, Rosell R, Valero P, Garcia-Giron C, Velasco A, et al. Epidermal growth factor receptor activating mutations in Spanish gefitinib-treated non-small-cell lung cancer patients. Ann Oncol. 2005. 16:1081–1086.20. Jackman DM, Yeap BY, Sequist LV, Lindeman N, Holmes AJ, Joshi VA, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006. 12:3908–3914.21. Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006. 12:6494–6501.22. Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007. 316:1039–1043.23. Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008. 14:2895–2899.24. Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008. 359:366–377.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of an EGFR mutation by PNA clamping method in lung carcinoid tumors
- Peptide Nucleic Acid Clamping Versus Direct Sequencing for the Detection of EGFR Gene Mutation in Patients with Non-small Cell Lung Cancer
- KRAS Mutation Detection in Non-small Cell Lung Cancer Using a Peptide Nucleic Acid-Mediated Polymerase Chain Reaction Clamping Method and Comparative Validation with Next-Generation Sequencing
- Comparative Analysis of Peptide Nucleic Acid (PNA)-Mediated Real-Time PCR Clamping and DNA Direct Sequencing for EGFR Mutation Detection
- Comparison of Direct Sequencing, PNA Clamping-Real Time Polymerase Chain Reaction, and Pyrosequencing Methods for the Detection of EGFR Mutations in Non-small Cell Lung Carcinoma and the Correlation with Clinical Responses to EGFR Tyrosine Kinase Inhibitor Treatment