Korean J Physiol Pharmacol.
2015 Jan;19(1):59-64. 10.4196/kjpp.2015.19.1.59.
Pectin Micro- and Nano-capsules of Retinyl Palmitate as Cosmeceutical Carriers for Stabilized Skin Transport
- Affiliations
-
- 1College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. jaehwi@cau.ac.kr
- 2Department of Food Science and Technology, Chung-Ang University, Anseong 456-756, Korea.
- 3Department of Chemical Engineering and Material Science, Chung-Ang University, Seoul 156-756, Korea.
- 4Department of Pharmacology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea.
- KMID: 2071820
- DOI: http://doi.org/10.4196/kjpp.2015.19.1.59
Abstract
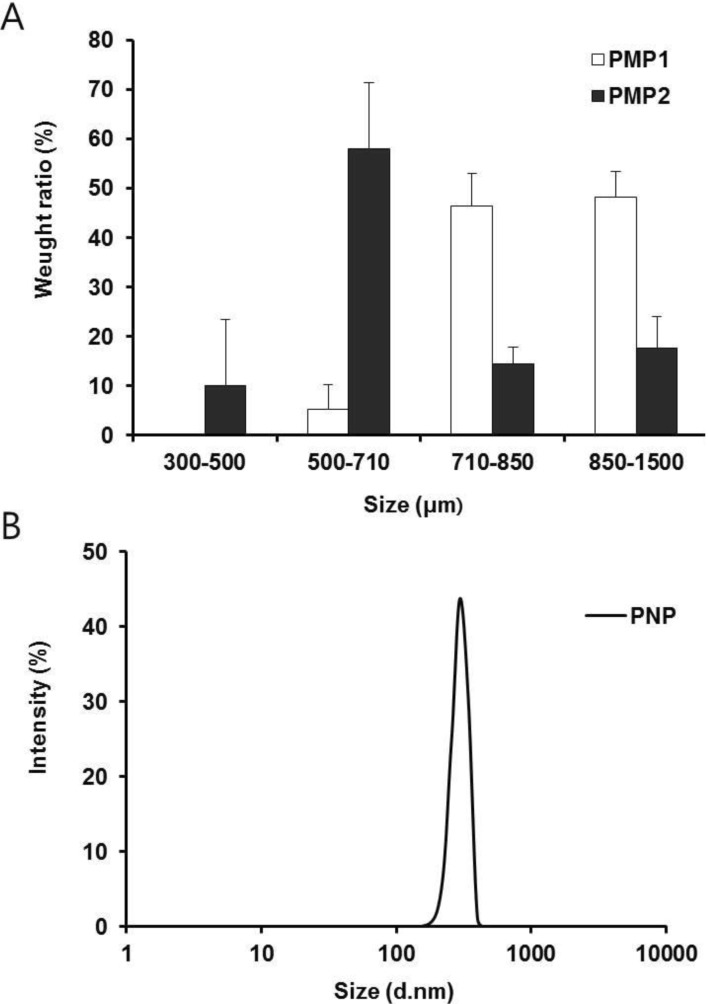
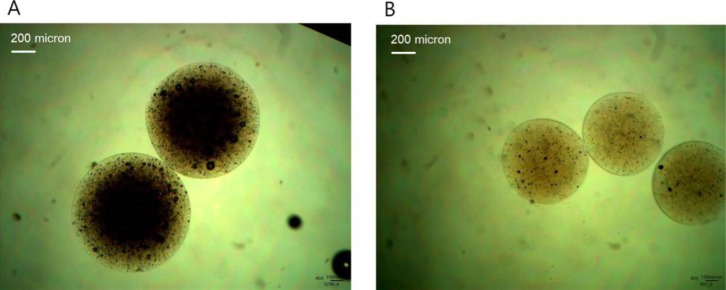
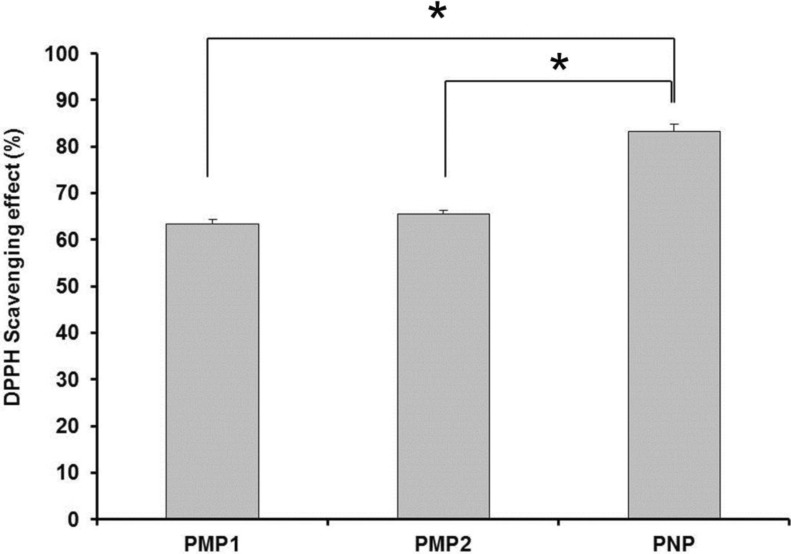
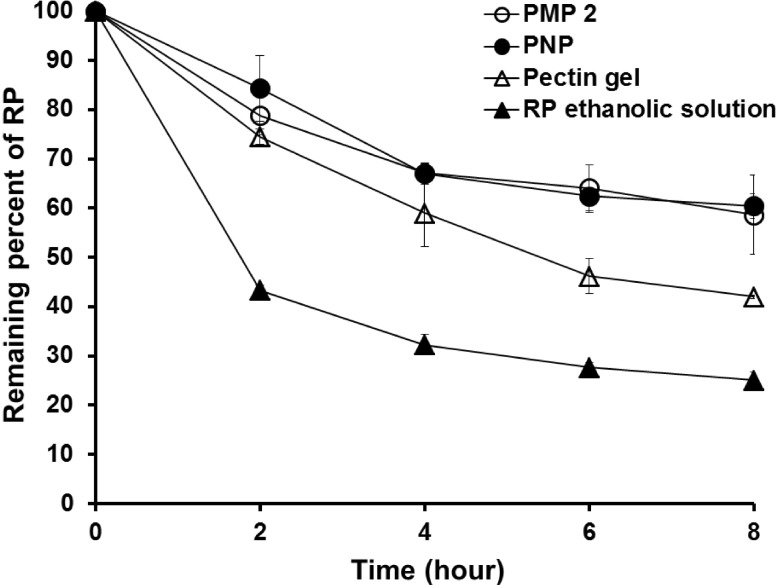
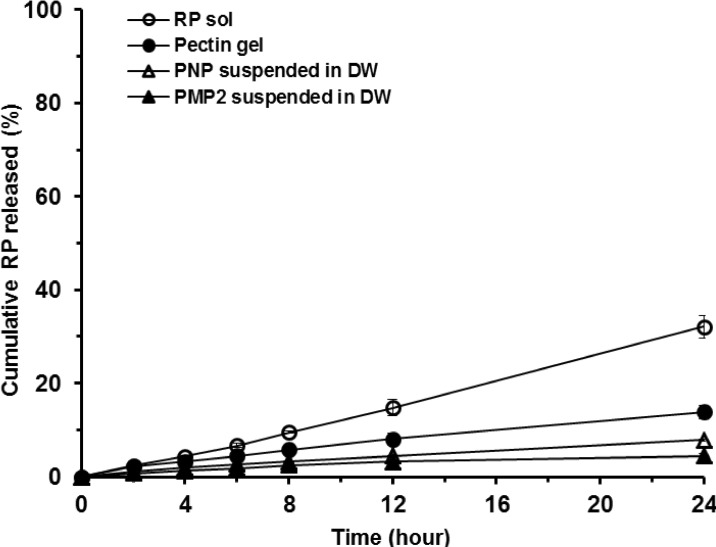
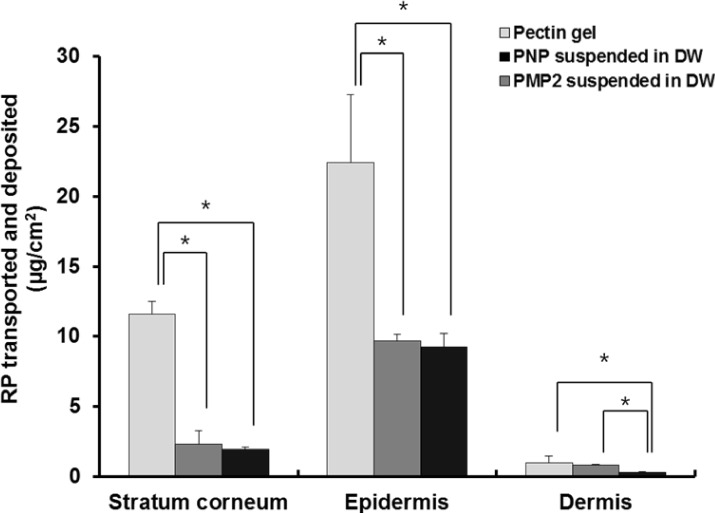
- Retinyl palmitate (RP)-loaded pectinate micro- and nano-particles (PMP and PNP) were designed for stabilization of RP that is widely used as an anti-wrinkle agent in anti-aging cosmeceuticals. PMP/PNP were prepared with an ionotropic gelation method, and anti-oxidative activity of the particles was measured with a DPPH assay. The stability of RP in the particles along with pectin gel and ethanolic solution was then evaluated. In vitro release and skin permeation studies were performed using Franz diffusion cells. Distribution of RP in each skin tissue (stratum corneum, epidermis, and dermis) was also determined. PMP and PNP could be prepared with mean particle size diameters of 593~843 mum (PMP) and 530 nm (i.e., 0.53 mum, PNP). Anti-oxidative activity of PNP was greater than PMP due largely to larger surface area available for PNP. The stability of RP in PMP and PNP was similar but much greater than RP in pectin bulk gels and ethanolic solution. PMP and PNP showed the abilities to constantly release RP and it could be permeated across the model artificial membrane and rat whole skin. RP was serially deposited throughout the skin layers. This study implies RP loaded PMP and PNP are expected to be advantageous for improved anti-wrinkle effects.
Keyword
MeSH Terms
Figure
Reference
-
1. Pena Ferreira MR, Costa PC, Bahia FM. Efficacy of antiwrinkle products in skin surface appearance: a comparative study using non-invasive methods. Skin Res Technol. 2010; 16:444–449. PMID: 20923458.
Article2. Hubinger JC. Determination of retinol, retinyl palmitate, and retinoic acid in consumer cosmetic products. J Cosmet Sci. 2009; 60:485–500. PMID: 19822106.
Article3. Boehnlein J, Sakr A, Lichtin JL, Bronaugh RL. Characterization of esterase and alcohol dehydrogenase activity in skin. Metabolism of retinyl palmitate to retinol (vitamin A) during percutaneous absorption. Pharm Res. 1994; 11:1155–1159. PMID: 7971717.4. Jee JP, Lim SJ, Park JS, Kim CK. Stabilization of all-trans retinol by loading lipophilic antioxidants in solid lipid nanoparticles. Eur J Pharm Biopharm. 2006; 63:134–139. PMID: 16527470.
Article5. Tolleson WH, Cherng SH, Xia Q, Boudreau M, Yin JJ, Wamer WG, Howard PC, Yu H, Fu PP. Photodecomposition and phototoxicity of natural retinoids. Int J Environ Res Public Health. 2005; 2:147–155. PMID: 16705812.
Article6. Carlotti ME, Rossatto V, Gallarate M. Vitamin A and vitamin A palmitate stability over time and under UVA and UVB radiation. Int J Pharm. 2002; 240:85–94. PMID: 12062504.
Article7. Carlotti ME, Rossatto V, Gallarate M, Trotta M, Debernardi F. Vitamin A palmitate photostability and stability over time. J Cosmet Sci. 2004; 55:233–252. PMID: 15264052.
Article8. Ro J, Kim Y, Kim H, Jang SB, Lee HJ, Chakma S, Jeong JH, Lee J. Anti-oxidative activity of pectin and its stabilizing effect on retinyl palmitate. Korean J Physiol Pharmacol. 2013; 17:197–201. PMID: 23776395.
Article9. Suh DC, Kim Y, Kim H, Ro J, Cho SW, Yun G, Choi SU, Lee J. Enhanced In Vitro Skin Deposition Properties of Retinyl Palmitate through Its Stabilization by Pectin. Biomol Ther (Seoul). 2014; 22:73–77. PMID: 24596625.
Article10. Sriamornsak P, Nunthanid J. Calcium pectinate gel beads for controlled release drug delivery: I. Preparation and in vitro release studies. J Microencapsul. 1998; 160:207–212.11. Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem. 1998; 62:1201–1204. PMID: 9692204.
Article12. Gray WA, Granada JF. Drug-coated balloons for the prevention of vascular restenosis. Circulation. 2010; 121:2672–2680. PMID: 20566965.
Article13. Alfadul SM, Elneshwy AA. Use of nanotechnology in food processing, packaging and safety review. Afr J Food Agric Nutr Dev. 2010; 10:2719–2739.14. Carlotti ME, Ugazio E, Sapino S, Peira E, Gallarate M. Photodegradation of retinol and anti-aging effectiveness of two commercial emulsions. J Cosmet Sci. 2006; 57:261–277. PMID: 16957807.15. Yoshida K, Sekine T, Matsuzaki F, Yanaki T, Yamaguchi M. Stability of vitamin A in oil-in-water-in-oil-type multiple emulsions. J Am Oil Chem Soc. 1999; 76:1–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-Oxidative Activity of Pectin and Its Stabilizing Effect on Retinyl Palmitate
- Enhanced In Vitro Skin Deposition Properties of Retinyl Palmitate through Its Stabilization by Pectin
- Skin Whitening Effects of Sanguisorba officinalis and Stichopus japonicus
- Anti-Melanogenic Potentials of Nanoparticles from Calli of Resveratrol-Enriched Rice against UVB-Induced Hyperpigmentation in Guinea Pig Skin
- Development of Micro/Nano Devices for Cancer Diagnosis