Korean J Physiol Pharmacol.
2012 Apr;16(2):113-118. 10.4196/kjpp.2012.16.2.113.
Effects of Protopanaxatriol-Ginsenoside Metabolites on Rat N-Methyl-D-Aspartic Acid Receptor-Mediated Ion Currents
- Affiliations
-
- 1Department of Physiology, College of Veterinary Medicine and Bio/Molecular Informatics Center, Konkuk University, Seoul 143-701, Korea. synah@konkuk.ac.kr
- 2Dominick P. Purpura Department of Neuroscience, Albert Einstein College of Medicine, NY 10461, USA.
- 3Life Science Division, KIST, Seoul 130-701, Korea.
- KMID: 2071756
- DOI: http://doi.org/10.4196/kjpp.2012.16.2.113
Abstract
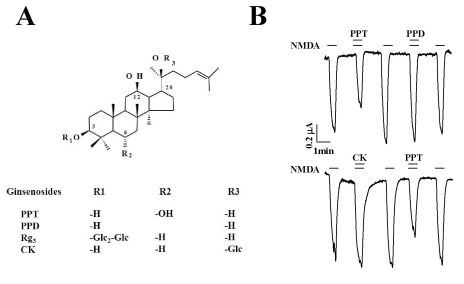
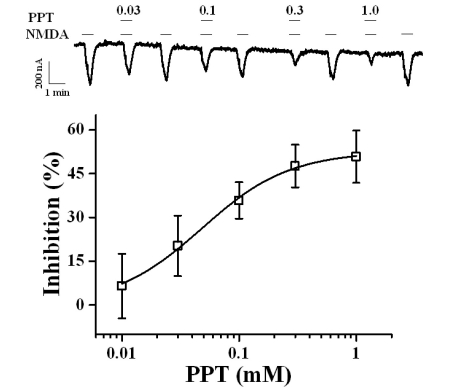
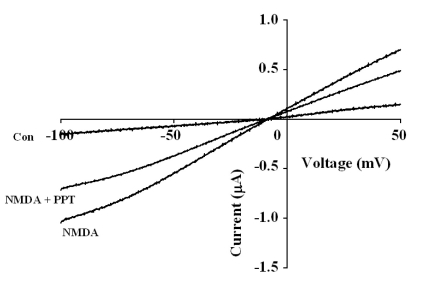
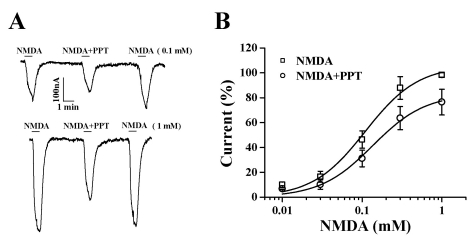
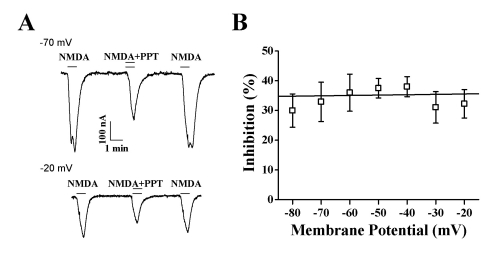
- Ginsenosides are low molecular weight glycosides found in ginseng that exhibit neuroprotective effects through inhibition of N-methyl-D-aspartic acid (NMDA) receptor channel activity. Ginsenosides, like other natural compounds, are metabolized by gastric juices and intestinal microorganisms to produce ginsenoside metabolites. However, little is known about how ginsenoside metabolites regulate NMDA receptor channel activity. In the present study, we investigated the effects of ginsenoside metabolites, such as compound K (CK), protopanaxadiol (PPD), and protopanaxatriol (PPT), on oocytes that heterologously express the rat NMDA receptor. NMDA receptor-mediated ion current (INMDA) was measured using the 2-electrode voltage clamp technique. In oocytes injected with cRNAs encoding NMDA receptor subunits, PPT, but not CK or PPD, reversibly inhibited INMDA in a concentration-dependent manner. The IC50 for PPT on INMDA was 48.1+/-4.6 microM, was non-competitive with NMDA, and was independent of the membrane holding potential. These results demonstrate the possibility that PPT interacts with the NMDA receptor, although not at the NMDA binding site, and that the inhibitory effects of PPT on INMDA could be related to ginseng-mediated neuroprotection.
MeSH Terms
Figure
Reference
-
1. Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991; 354:31–37. PMID: 1834949.
Article2. Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994; 17:31–108. PMID: 8210177.
Article3. Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002; 25:571–577. PMID: 12392932.
Article5. Soundarapandian MM, Tu WH, Peng PL, Zervos AS, Lu Y. AMPA receptor subunit GluR2 gates injurious signals in ischemic stroke. Mol Neurobiol. 2005; 32:145–155. PMID: 16215279.
Article6. Nah SY. Ginseng: recent advances and trends. Korean J Ginseng Sci. 1997; 21:1–12.7. Nah SY, Kim DH, Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007; 13:381–404. PMID: 18078425.
Article8. Kim S, Ahn K, Oh TH, Nah SY, Rhim H. Inhibitory effect of ginsenosides on NMDA receptor-mediated signals in rat hippocampal neurons. Biochem Biophys Res Commun. 2002; 296:247–254. PMID: 12163009.
Article9. Kim S, Kim T, Ahn K, Park WK, Nah SY, Rhim H. Ginsenoside Rg3 antagonizes NMDA receptors through a glycine modulatory site in rat cultured hippocampal neurons. Biochem Biophys Res Commun. 2004; 323:416–424. PMID: 15369768.10. Lee JH, Jeong SM, Kim JH, Lee BH, Yoon IS, Lee JH, Choi SH, Lee SM, Park YS, Lee JH, Kim SS, Kim HC, Lee BY, Nah SY. Effects of ginsenosides and their metabolites on voltage-dependent Ca2+ channel subtypes. Mol Cells. 2006; 21:52–62. PMID: 16511347.11. Choi SH, Lee JH, Pyo MK, Lee BH, Shin TJ, Hwang SH, Kim BR, Lee SM, Oh JW, Kim HC, Bae CS, Rhim H, Nah SY. Mutations Leu427, Asn428, and Leu431 residues within transmembrane domain-I-segment 6 attenuate ginsenoside-mediated L-type Ca2+ channel current inhibitions. Biol Pharm Bull. 2009; 32:1224–1230. PMID: 19571390.12. Lee JH, Jeong SM, Kim JH, Lee BH, Yoon IS, Lee JH, Choi SH, Kim DH, Rhim H, Kim SS, Kim JI, Jang CG, Song JH, Nah SY. Characteristics of ginsenoside Rg3-mediated brain Na+ current inhibition. Mol Pharmacol. 2005; 68:1114–1126. PMID: 16014805.13. Lee JH, Lee BH, Choi SH, Yoon IS, Shin TJ, Pyo MK, Lee SM, Kim HC, Nah SY. Involvement of batrachotoxin binding sites in ginsenoside-mediated voltage-gated Na+ channel regulation. Brain Res. 2008; 1203:61–67. PMID: 18321475.14. Hasegawa H, Suzuki R, Nagaoka T, Tezuka Y, Kadota S, Saiki I. Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid-conjugate of protopanaxatriol. Biol Pharm Bull. 2002; 25:861–866. PMID: 12132658.
Article15. Wakabayashi C, Murakami K, Hasegawa H, Murata J, Saiki I. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun. 1998; 246:725–730. PMID: 9618279.
Article16. Lee BH, Jeong SM, Lee JH, Kim JH, Yoon IS, Lee JH, Choi SH, Lee SM, Chang CG, Kim HC, Han Y, Paik HD, Kim Y, Nah SY. Quercetin inhibits the 5-hydroxytryptamine type 3 receptor-mediated ion current by interacting with pre-transmembrane domain I. Mol Cells. 2005; 20:69–73. PMID: 16258243.17. Zheng X, Zhang L, Wang AP, Bennett MV, Zukin RS. Ca2+ influx amplifies protein kinase C potentiation of recombinant NMDA receptors. J Neurosci. 1997; 17:8676–8686. PMID: 9348336.18. Durand GM, Bennett MV, Zukin RS. Splice variants of the N-methyl-D-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc Natl Acad Sci USA. 1993; 90:6731–6735. PMID: 8341692.
Article19. Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992; 256:1217–1221. PMID: 1350383.
Article20. Choi SH, Shin TJ, Hwang SH, Lee BH, Kang J, Kim HJ, Oh JW, Bae CH, Lee SH, Nah SY. Differential effects of ginsenoside metabolites on HERG K+ channel currents. J Ginseng Res. 2011; 35:191–199.21. Lee BH, Jeong SM, Lee JH, Kim DH, Kim JH, Kim JI, Shin HC, Lee SM, Nah SY. Differential effect of ginsenoside metabolites on the 5-HT3A receptor-mediated ion current in Xenopus oocytes. Mol Cells. 2004; 17:51–56. PMID: 15055527.22. Lee JH, Jeong SM, Lee BH, Kim DH, Kim JH, Kim JI, Lee SM, Nah SY. Differential effect of bovine serum albumin on ginsenoside metabolite-induced inhibition of alpha3beta4 nicotinic acetylcholine receptor expressed in Xenopus oocytes. Arch Pharm Res. 2003; 26:868–873. PMID: 14609137.23. Chapman AG. Glutamate and epilepsy. J Nutr. 2000; 130(4S Suppl):1043S–1045S. PMID: 10736378.
Article24. Ikonomidou C, Turski L. Neurodegenerative disorders: clues from glutamate and energy metabolism. Crit Rev Neurobiol. 1996; 10:239–263. PMID: 8971131.
Article25. Lee HG, Zhu X, Ghanbari HA, Ogawa O, Raina AK, O'Neill MJ, Perry G, Smith MA. Differential regulation of glutamate receptors in Alzheimer's disease. Neurosignals. 2002; 11:282–292. PMID: 12566929.
Article26. Qian T, Cai Z, Wong RN, Mak NK, Jiang ZH. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J Chromatogr B Analyt Technol Biomed Life Sci. 2005; 816:223–232.
Article27. Zhao Q, Zheng X, Jiang J, Zhou H, Hu P. Determination of ginsenoside Rg3 in human plasma and urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010; 878:2266–2273.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory Effects of Ginsenoside Metabolites, Compound K and Protopanaxatriol, on GABAC Receptor-Mediated Ion Currents
- Enhancement of GluN2B Subunit-Containing NMDA Receptor Underlies Serotonergic Regulation of Long-Term Potentiation after Critical Period in the Rat Visual Cortex
- Effect of propofol on ion channels in acutely dissociated dorsal raphe neuron of Sprague-Dawley rats
- Differential effect of homocysteic acid and cysteic acid on changes of inositol phosphates and (Ca2+)i in rat cerebellar granule cells
- Resveratrol attenuates N-methyl-D-aspartate receptor-mediated activation in substantia gelatinosa neurons of trigeminal subnucleus caudalis in mice