Korean J Physiol Pharmacol.
2011 Oct;15(5):307-312. 10.4196/kjpp.2011.15.5.307.
Roles of Non-cholinergic Intrapancreatic Nerves, Serotonergic Nerves, on Pancreatic Exocrine Secretion in the Isolated Perfused Rat Pancreas
- Affiliations
-
- 1Department of Physiology, College of Nursing, Yanbian University, Yanji 133000, China.
- 2Department of Physiology, College of Medicine, allym University, Chuncheon 200-702, Korea. yylee@hallym.ac.kr
- 3Department of Occupational Therapy, Dongnam Health College, Suwon 440-714, Korea.
- 4Department of Veterinary Lab. Animal Medicine & Science, College of Animal Resource Science, Chuncheon 200-701, Korea.
- 5Department of Neurobiology, School of Medicine, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 2071734
- DOI: http://doi.org/10.4196/kjpp.2011.15.5.307
Abstract
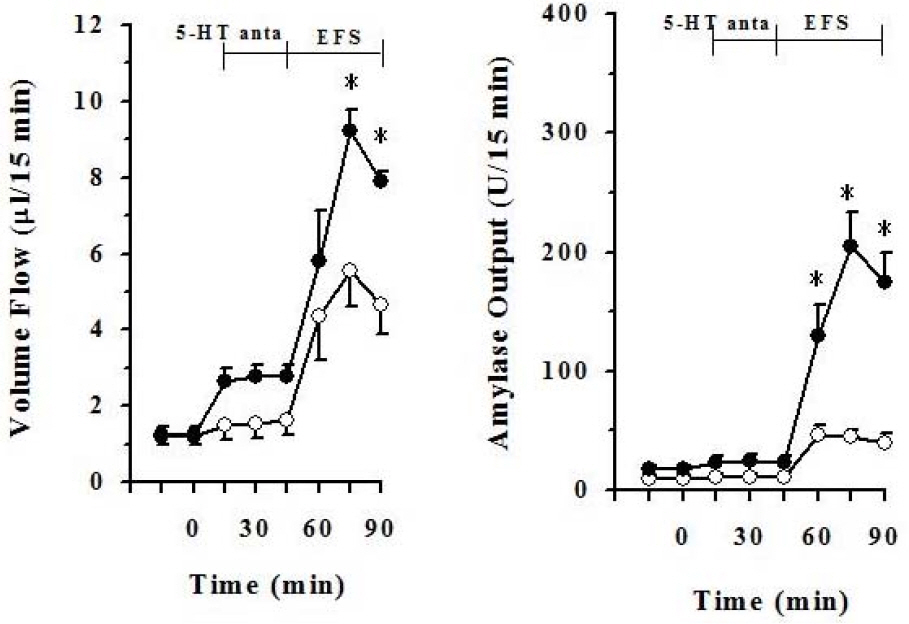
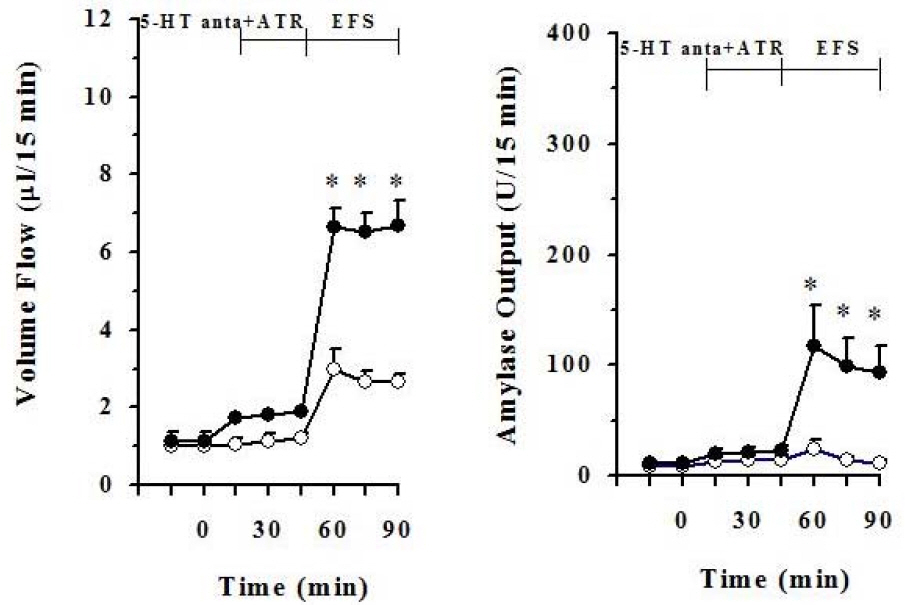
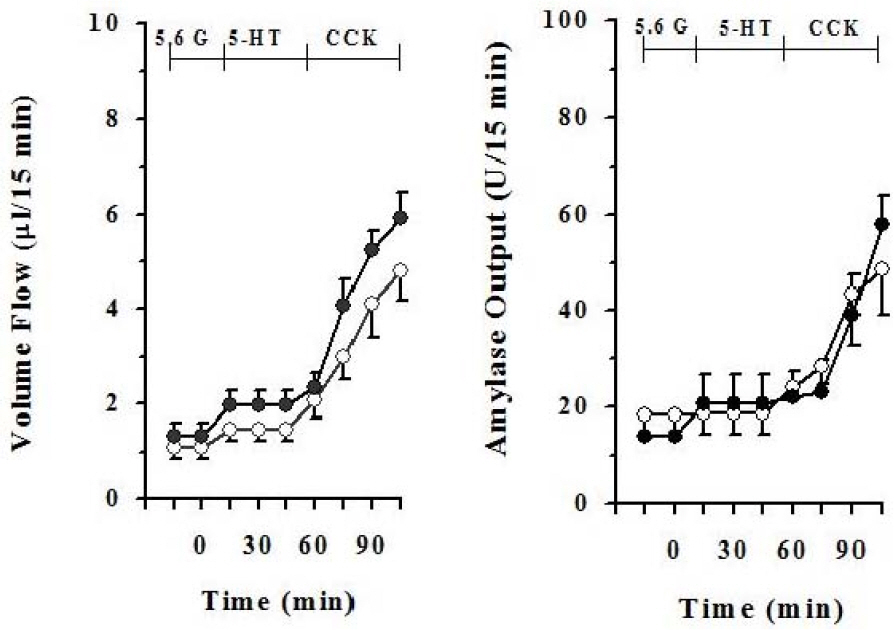
- It has been rereported that axons which display 5-hydroxytryptamine (5-HT) immunoreactivity are abundant in the pancreas and the majority of serotonergic axons terminate within intrapancreatic ganglia, islet and acini. This histological result strongly suggests that intrapancreatic serotonergic nerves could affect to the pancreatic endocrine and exocrine secretion. Thus, this study was aimed to investigate whether intrapancreatic serotonergic nerves could affect pancreatic exocrine secretion and an action mechanism of the intrapancreatic serotonergic nerves. The rats were anesthetized with a single injection of urethane. The median line and the abdominal aorta was carefully dissected and cannulated with PE-50 tubing just above the celiac artery, and then tightly ligated just below the superior mesenteric artery. The pancreatic duct was also cannulated with Tygon microbore tubing. With the addition of serotonin, pancreatic volume flow and amylase output were significantly inhibited electrical field stimulation (EFS). On the other hand, pancreatic volume flow and amylase output were significantly elevated in EFS with the addition of spiperone. EFS application, however, pancreatic volume flow and amylase output had no significant change in cholecystokinin (CCK) alone when serotonin was applied under a 5.6 mM glucose background. Pancreatic volume flow and amylase output under 18 mM glucose background were significantly elevated in CCK plus serotonin than in CCK alone. These data suggest that intrapancreatic serotonergic nerves play an inhibitory role in pancreatic exocrine secretion and an important role in the insulin action or release.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Age-associated changes in pancreatic exocrine secretion of the isolated perfused rat pancreas
Zheng-er Jiang, ChengZhe Jiang, Baihui Chen, Chin Su Koh, Jun-Hwan Yong, Dae-Hun Park, Moo-Ho Won, Yun-Lyul Lee
Lab Anim Res. 2013;29(1):19-26. doi: 10.5625/lar.2013.29.1.19.
Reference
-
References
1. Michaloudi EC, Papadopoulos GC. Atlas of the serotonin-containing cell bodies and fibers in the central nervous system of the hedgehog. J Hirnforsch. 1995; 36:77–100.2. Tack JF, Janssens J, Vantrappen G, Wood JD. Actions of 5-hydroxytryptamine on myenteric neurons in guinea pig gastric antrum. Am J Physiol. 1992; 263:G838–846.
Article3. Spiller RC. Effects of serotonin on intestinal secretion and motility. Curr Opin Gastroenterol. 2001; 17:99–103.
Article4. Kirchgessner AL, Liu MT, Raymond JR, Gershon MD. Identification of cells that express 5-hydroxytryptamine1A receptors in the nervous systems of the bowel and pancreas. J Comp Neurol. 1996; 364:439–455.5. Niebergall-Roth E, Singer MV. Central and peripheral neural control of pancreatic exocrine secretion. J Physiol Pharmacol. 2001; 52:523–538.6. Park HJ, Lee YL, Kwon HY. Effects of pancreatic polypeptide on insulin action in exocrine secretion of isolated rat pancreas. J Physiol. 1993; 463:421–429.
Article7. Nelson MT, Debas HT, Mulvihill SJ. Vagal stimulation of rat exocrine pancreatic secretion occurs via multiple mediators. Gastroenterology. 1993; 105:221–228.
Article8. Park HS, Park IS, Lee YL, Kwon HY, Park HJ. Effects of intrapancreatic neuronal activation on cholecystokinin-induced exocrine secretion of isolated perfused rat pancreas. Pflugers Arch. 1999; 437:511–516.
Article9. Park HS, Kwon HY, Lee YL, Chey WY, Park HJ. Role of GRP-ergic neurons in secretin-evoked exocrine secretion in isolated rat pancreas. Am J Physiol Gastrointest Liver Physiol. 2000; 278:G557–562.10. Park HS, Lee YL, Kwon HY, Chey WY, Park HJ. Significant cholinergic role in secretin-stimulated exocrine secretion in isolated rat pancreas. Am J Physiol. 1998; 274:G413–418.11. Hüchtebrock HJ, Niebel W, Singer MV, Forssmann WG. Intrinsic pancreatic nerves after mechanical denervation of the extrinsic pancreatic nerves in dogs. Pancreas. 1991; 6:1–8.
Article12. Kirchgessner AL, Gershon MD. Innervation of the pancreas by neurons in the gut. J Neurosci. 1990; 10:1626–1642.
Article13. Rick W, Stegbauer H. a-Amylase; Measurement of reducing groups. Methods of Enzymatic Analysis. 2nd ed.Weinheim: Verlag Chemie;1974. p. 885–915.14. Masuda M, Miyasaka K, Funakoshi A. Involvement of 5-hydroxytryptamine (5-HT)3 receptor mechanisms in regulation of basal pancreatic secretion in conscious rats. J Auton Nerv Syst. 1997; 62:58–62.
Article15. Nagain-Domaine C, Presset O, Chariot J, Rozé C. Effect of sumatriptan on external pancreatic secretion and its interaction with endogenous norepinephrine in the rat. Pancreas. 1999; 19:56–61.
Article16. Li JP, Chang TM, Chey WY. Roles of 5-HT receptors in the release and action of secretin on pancreatic secretion in rats. Am J Physiol Gastrointest Liver Physiol. 2001; 280:G595–602.
Article17. Coulie B, Tack J, Bouillon R, Peeters T, Janssens J. 5-Hydroxytryptamine-1 receptor activation inhibits endocrine pancreatic secretion in humans. Am J Physiol. 1998; 274:E317–320.18. Lifson N, Lassa CV, Dixit PK. Relation between blood flow and morphology in islet organ of rat pancreas. Am J Physiol. 1985; 249:E43–48.
Article19. Park HJ, Lee YL. Effect of reserpine on pancreatic exocrine secretion induced by mesencephalic reticular stimulation in rats. Korean J Physiol. 1988; 22:101–109.20. Lee KY, Lee YL, Kim CD, Chang TM, Chey WY. Mechanism of action of insulin on pancreatic exocrine secretion in perfused rat pancreas. Am J Physiol. 1994; 267:G207–212.
Article21. Lee YL, Kwon HY, Park HS, Lee TH, Park HJ. The role of insulin in the interaction of secretin and cholecystokinin in exocrine secretion of the isolated perfused rat pancreas. Pancreas. 1996; 12:58–63.
Article22. Peschke E, Peschke D, Hammer T, Csernus V. Influence of melatonin and serotonin on glucose-stimulated insulin release from perifused rat pancreatic islets in vitro. J Pineal Res. 1997; 23:156–163.
Article23. Choi KJ, Cho DS, Kim JY, Kim BJ, Lee KM, Kim SH, Kim DK, Kim SH, Park HS. Ca2+-induced Ca2+ release from internal stores in INS-1 rat insulinoma cells. Korean J Physiol Pharmacol. 2011; 15:53–59.24. Holst JJ. Neural regulation of pancreatic exocrine function. Go VLW, editor. ed,. The Pancreas: Biology, Pathobiology, and Dis-ease. 2nd ed.New York: Raven Press;1993. p. 381–402.25. Adeghate E, Ponery AS, Pallot D, Parvez SH, Singh J. Distribution of serotonin and its effect on insulin and glucagon secretion in normal and diabetic pancreatic tissues in rat. Neuro Endocrinol Lett. 1999; 20:315–322.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A role of endogenous somatostatin in exocrine secretion induced by intrapancreatic cholinergic activation
- Mechanism of action of pancreatic polypeptide (PP) on pancreatic exocrine secretion in isolated rat pancreas
- Roles of Gonadal Steroids on Exocrine Secretion of Isolated Perfused Rat Pancreas
- Effects of gamma-Aminobutyric Acid on Intrinsic Cholinergic Action in Exocrine Secretion of Isolated, Perfused Rat Pancreas
- Effects of GABA on pancreatic exocrine secretion of rats