Korean J Physiol Pharmacol.
2009 Aug;13(4):327-335. 10.4196/kjpp.2009.13.4.327.
Effects of Losartan on Catecholamine Release in the Isolated Rat Adrenal Gland
- Affiliations
-
- 1Department of Family Medicine, Eulji University Hospital, Daejeon 302-799,.
- 2DNA Repair Research Center, Chosun University College of Medicine, Gwangju 501-759, Korea.
- 3Department of Pharmacology, Chosun University College of Medicine, Gwangju 501-759, Korea. dylim@chosun.ac.kr
- KMID: 2071689
- DOI: http://doi.org/10.4196/kjpp.2009.13.4.327
Abstract
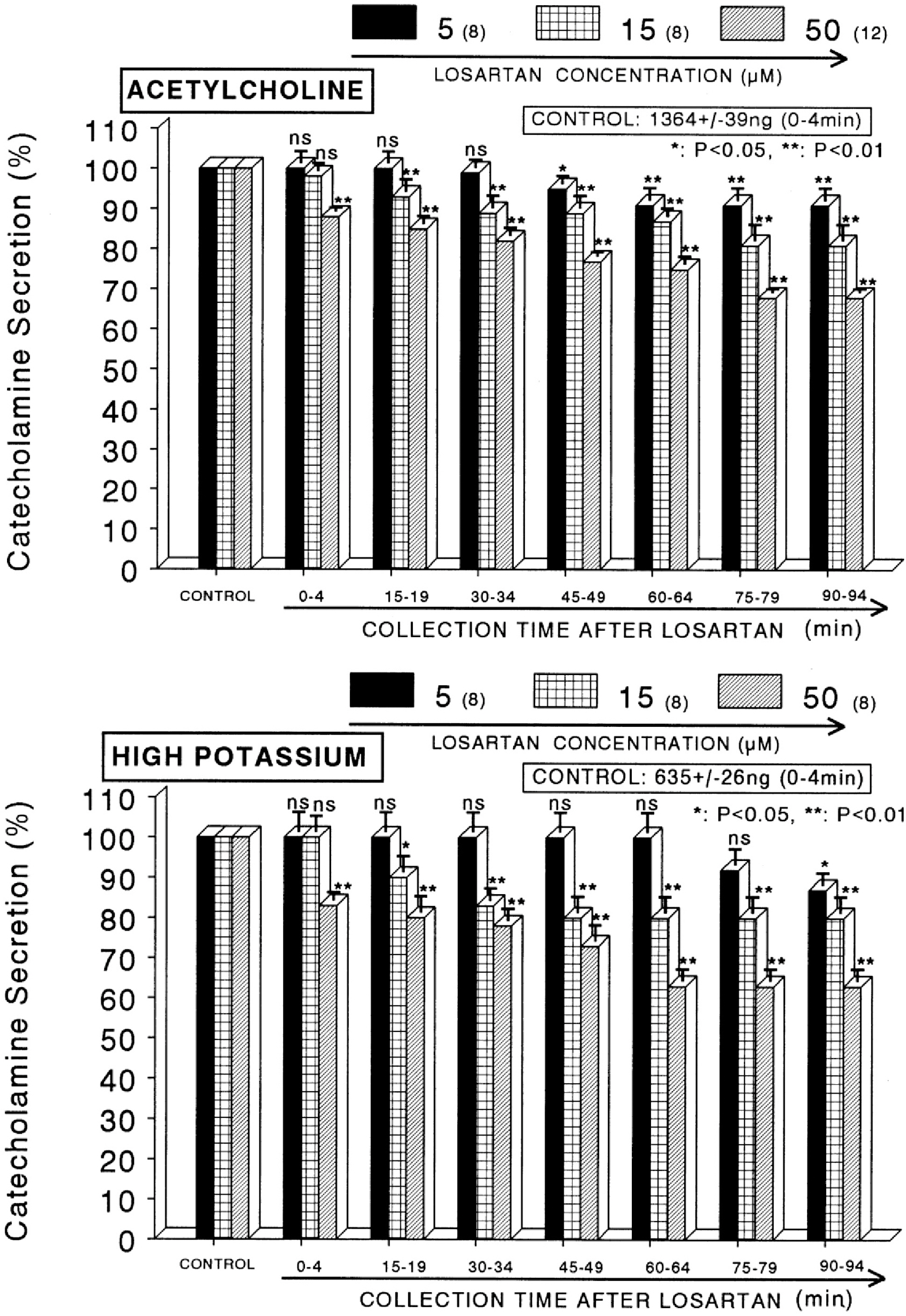
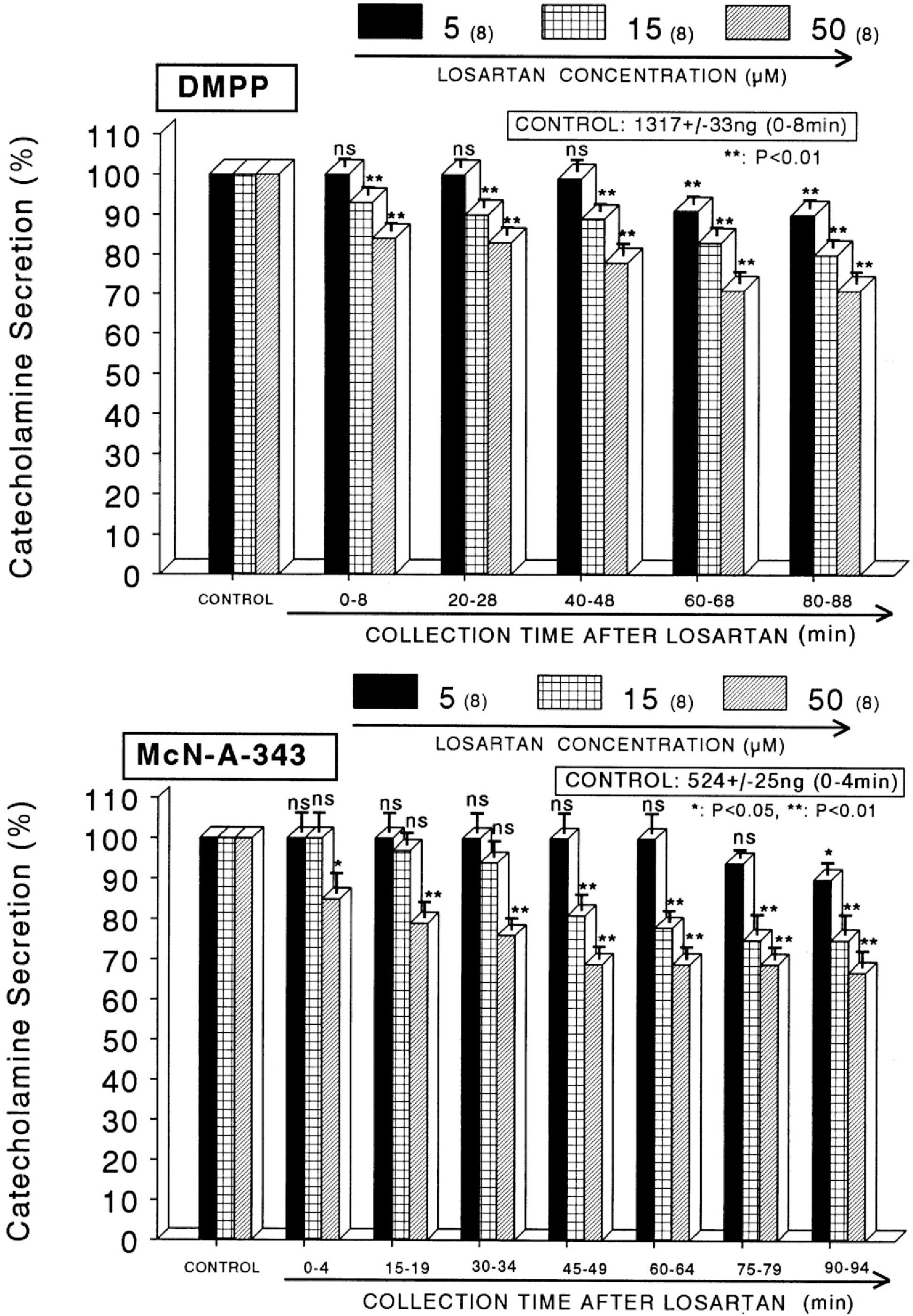
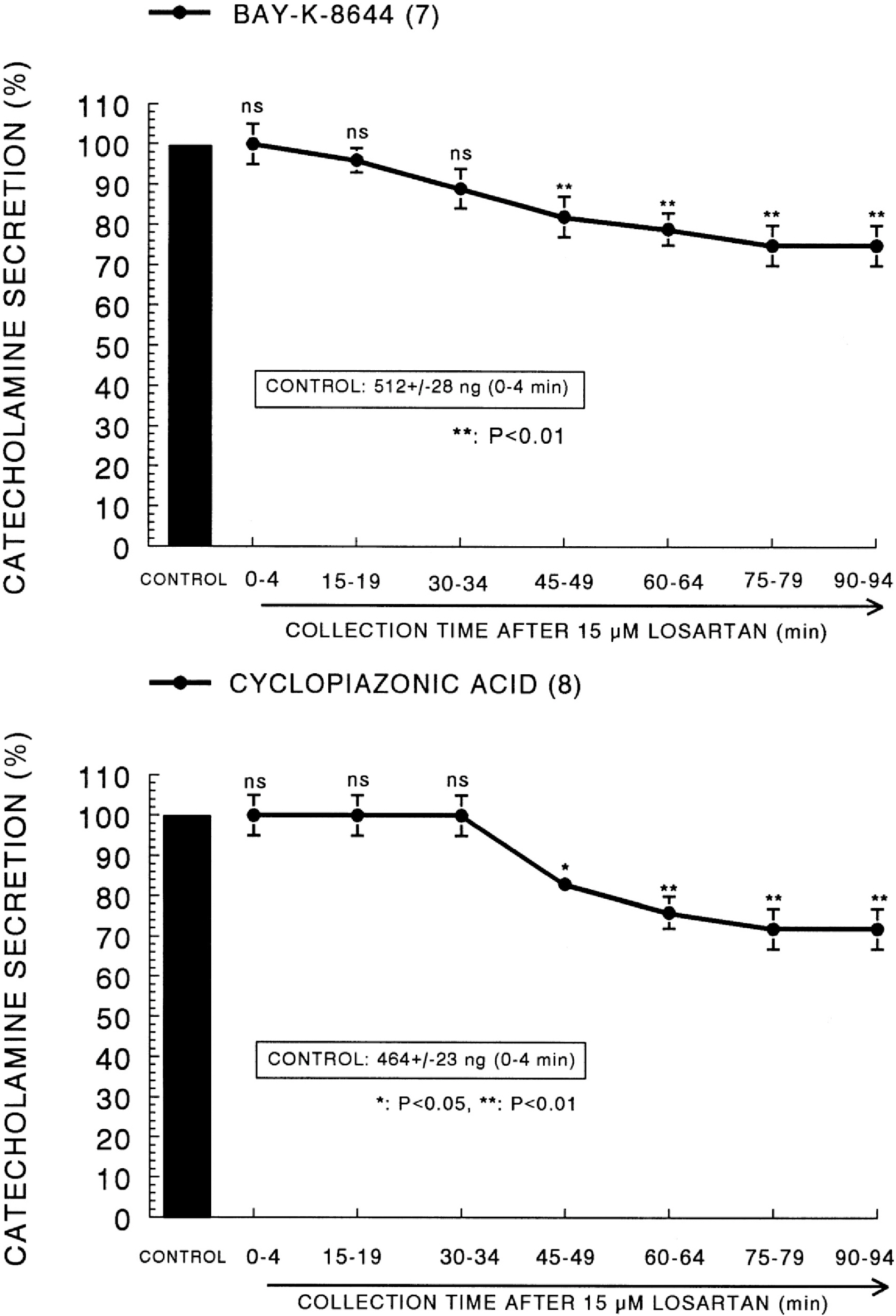
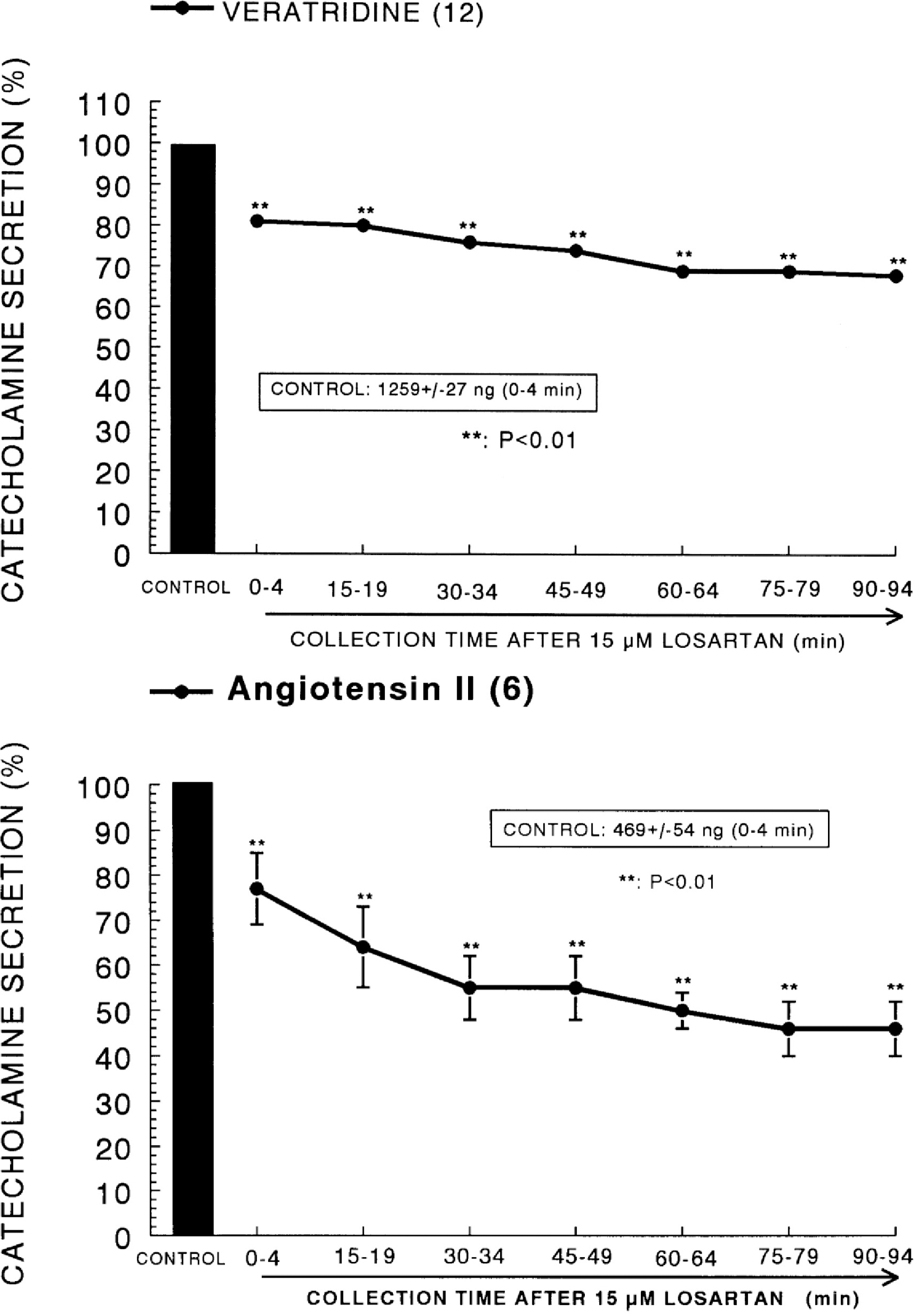
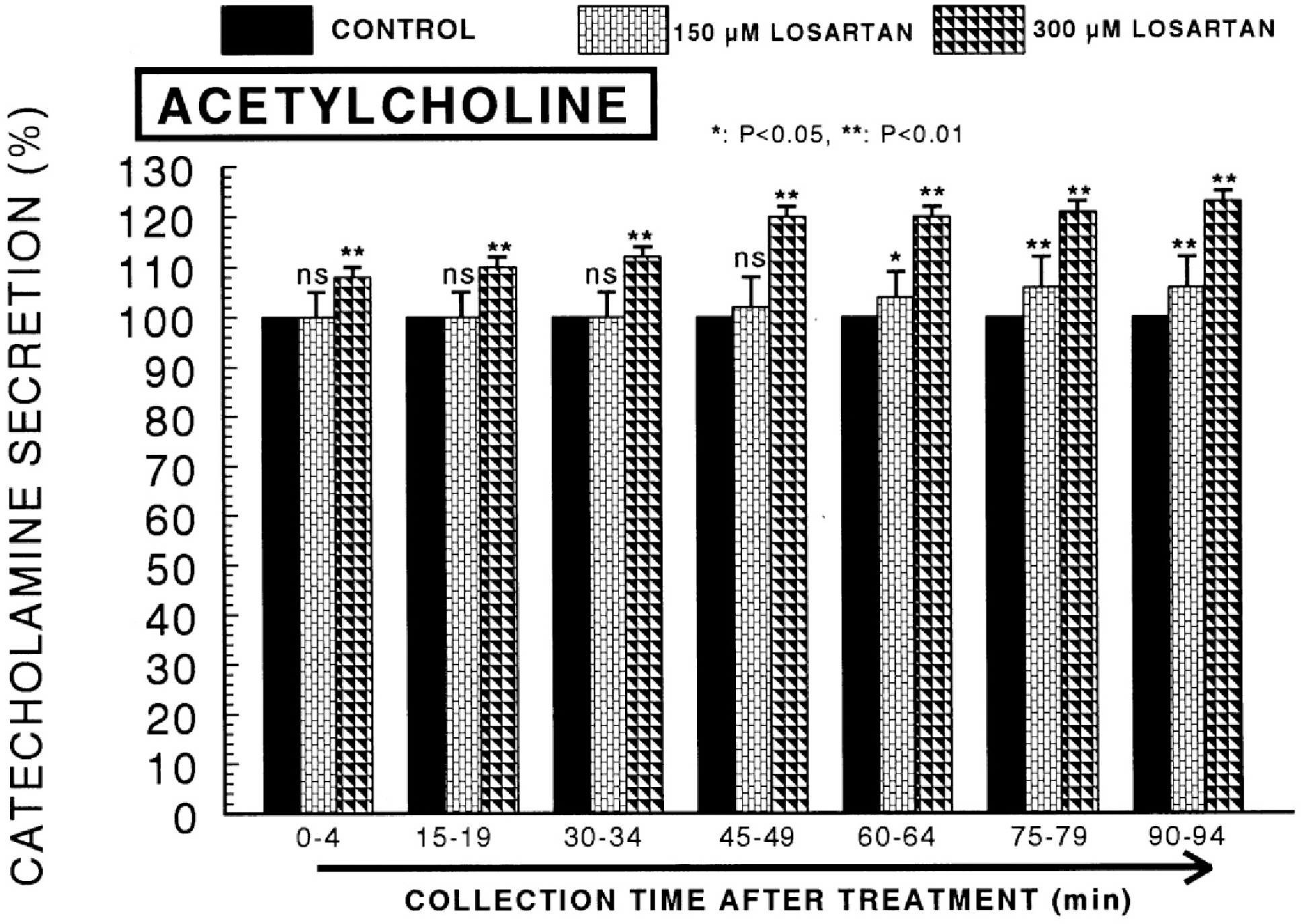
- The aim of this study was to determine whether losartan, an angiotensin II (Ang II) type 1 (AT1) receptor could influence the CA release from the isolated perfused model of the rat adrenal medulla. Losartan (5~50 micrometer) perfused into an adrenal vein for 90 min produced dose- and time-dependent inhibition of the CA secretory responses evoked by ACh (5.32 mM), high K+ (56 mM, a direct membrane depolarizer), DMPP (100 micrometer) and McN-A-343 (100 micrometer). Losartan failed to affect basal CA output. Furthermore, in adrenal glands loaded with losartan (15 micrometer) for 90 min, the CA secretory responses evoked by Bay-K-8644 (10 micrometer, an activator of L-type Ca2+ channels), cyclopiazonic acid (10 micrometer, an inhibitor of cytoplasmic Ca2+-ATPase), veratridine (100 micrometer, an activator of Na+ channels), and Ang II (100 nM) were markedly inhibited. However, at high concentrations (150~300 micrometer), losartan rather enhanced the CA secretion evoked by ACh. Collectively, these experimental results suggest that losartan at low concentrations inhibits the CA secretion evoked by cholinergic stimulation (both nicotininc and muscarinic receptors) as well as by membrane depolarization from the rat adrenal medulla, but at high concentration it rather inhibits ACh-evoked CA secretion. It seems that losartan has a dual action, acting as both agonist and antagonist to nicotinic receptors of the rat adrenal medulla, which might be dependent on the concentration. It is also thought that this inhibitory effect of losartan may be mediated by blocking the influx of both Na+ and Ca2+ into the rat adrenomedullary chromaffin cells as well as by inhibiting the Ca2+ release from the cytoplasmic calcium store, which is thought to be relevant to the AT1 receptor blockade, in addition to its enhancement of the CA release.
MeSH Terms
-
(4-(m-Chlorophenylcarbamoyloxy)-2-butynyl)trimethylammonium Chloride
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Adrenal Glands
Adrenal Medulla
Angiotensin II
Animals
Calcium
Chromaffin Cells
Cytoplasm
Dimethylphenylpiperazinium Iodide
Indoles
Losartan
Membranes
Rats
Receptors, Nicotinic
Veins
Veratridine
(4-(m-Chlorophenylcarbamoyloxy)-2-butynyl)trimethylammonium Chloride
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Angiotensin II
Calcium
Dimethylphenylpiperazinium Iodide
Indoles
Losartan
Receptors, Nicotinic
Veratridine
Figure
Cited by 2 articles
-
Influence of Fimasartan (a Novel AT1 Receptor Blocker) on Catecholamine Release in the Adrenal Medulla of Spontaneously Hypertensive Rats
Hyo-Jeong Lim, Seog-Ki Lee, Dong-Yoon Lim
Korean J Physiol Pharmacol. 2013;17(1):99-109. doi: 10.4196/kjpp.2013.17.1.99.Influence of PD 123319 (AT2-Receptor Antagonist) on Catecholamine Secretion in the Perfused Rat Adrenal Medulla
Soon-Pyo Hong, Bhandary Bidur, Mee-Sung Choi, Young-Hwan Seo, Dong-Yoon Lim
J Korean Soc Hypertens. 2013;19(1):23-38. doi: 10.5646/jksh.2013.19.1.23.
Reference
-
Aguilera G., Kiss A., Luo X. Increased expression of type 1 angiotensin II receptors in the hypothalamic paraventricular nucleus following stress and glucocorticoid administration. J Neuroendocrinol. 7:775–783. 1995.
ArticleAnton AH., Sayre DF. A study of the factors affecting the aluminum oxidetrihydroxy indole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 138:360–375. 1962.Armando I., Carranza A., Nishimura Y., Hoe KL., Barontini M., Terron JA., Falcon-Neri A., Ito T., Jourio AV., Saavedra JM. Peripheral administration of and angiotensin II AT1 receptor antagonist decreases the hypothalamic-pituitary-adrenal response to isolation stress. Endocrinology. 142:3880–3889. 2001.Armando I., Jezova M., Bregonzio C., Baiardi G., Saavedra JM. Angiotensin II AT1 and AT2 receptor types regulate basal and stress-induced adrenomedullary catecholamine production through transcriptional regulation of tyrosine hydroxylase. Ann NY Acad Sci. 1018:302–309. 2004.Barber MN., Sampey DB., Widdop RE. AT(2) receptor stimulation enhances antihypertensive effect of AT(1) receptor antagonist in hypertensive rats. Hypertension. 34:1112–1116. 1999.Bunn SJ., Marley PD. Effects of angiotensin II on cultured, bovine adrenal medullary cells. Neuropeptides. 13:121–132. 1989.
ArticleCastrén E., Saavedra JM. Repeated stress increases the density of angiotensin II binding sites in the rat paraventricular nucleus and subfornical organ. Endocrinology. 122:370–372. 1988.Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 72:15–48. 1992.
ArticleCatterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 26:13–25. 2000.Challiss RA., Jones JA., Owen PJ., Boarder MR. Changes in inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate mass accumulations in cultured adrenal chromaffin cells in response to bradykinin and histamine. J Neurochem. 56:1083–1086. 1991.
ArticleCheek TR., O'Sullivan AJ., Moreton RB., Berridge MJ., Burgoyne RD. Spatial localization of the stimulus-induced rise in cyrosolic Ca2+ in bovine adrenal chromaffin cells: Distinct nicotinic and muscarinic patterns. FEBS Lett. 247:429–434. 1989.Critchley L., Ding B., Fok B., Wang D., Tomlinson B., James A., Thomas GN., Critchley J. The effects of candesartan and ramipril on adrenal catecholamine release in anaesthetized dogs. Eur J Pharmacol. 489:67–75. 2004.
ArticleDendorfer A., Raasch W., Tempel K., Dominiak P. Interactions between the renin-angiotensin system (RAS) and the sympathetic system. Basic Res Cardiol. 93:24–29. 1998.
ArticleDunn LA., Holz RW. Catecholamine secretion from digitonin-treated adrenal medullary chromaffin cells. J Biol Chem. 258:4989–4993. 1983.
ArticleGarcia AG., Sala F., Reig JA., Viniegra S., Frias J., Fonteriz R., Gandia L. Dihydropyridine Bay-K-8644 activates chromaffin cell calcium channels. Nature. 309:69–71. 1984.
ArticleGhosh A., Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 268:239–247. 1995.
ArticleGoeger DE., Riley RT. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 38:3995–4003. 1989.Hammer R., Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 31:2992–2998. 1982.Han HJ., Park SH., Koh HJ., Taub M. Mechanism of regulation of Na+ transport by angiotensin II in primary renal cells. Kidney Int. 57:2457–2467. 2000.Hano T., Mizukoshi M., Baba A., Nakamura N., Nishio I. Angiotensin II subtype 1 receptor modulates epinephrine release from isolated rat adrenal gland. Blood Press. 5:105–108. 1994.Holz RW., Senter RA., Frye RA. Relationship between Ca2+ uptake and catecholamine secretion in primary dissociated cultures of adrenal modulla. J Neurochem. 39:635–640. 1982.Israel A., Strömberg C., Tsutsumi K., Garrido MR., Torres M., Saavedra JM. Angiotensin II receptor subtypes and phosphoinositide hydrolysis in rat adrenal medulla. Brain Res Bull. 38:441–446. 1995.
ArticleKubo T., Numakura H., Endo S., Hagiwara Y., Fukumori R. Angiotensin receptor blockade in the anterior hypothalamic area inhibits stress-induced pressor responses in rats. Brain Res Bull. 56:569–574. 2001.
ArticleLim DY., Kim CD., Ahn KW. Influence of TMB-8 on secretion of catecholamines from the perfused rat adrenal glands. Arch Pharm Res. 15:115–125. 1992.
ArticleLivett BG., Marley PD. Non cholinergic control of adrenal catecholamine secretion. J Anat. 183:277–289. 1993.Martineau D., Lamouche S., Briand R., Yamaguchi N. Functional involvement of angiotensin AT2 receptor in adrenal catecholamine secretion in vivo. Can J Physiol Pharmacol. 77:367–374. 1999.Martineau D., Yamaguchi N., Briand R. Inhibition by BMS 186295, a selective nonpeptide AT1 antagonist, of adrenal catecholamine release induced by angiotensin II in the dog. in vivo. Can J Physiol Pharmacol. 73:459–464. 1995.McGehee DS., Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 57:521–546. 1995.
ArticleNahmod VE., Finkielman S., Benarroch EE., Pirola CJ. Angiotensin regulates release and synthesis of serotonin in brain. Science. 202:1091–1093. 1978.
ArticlePhillips MI., Speakman EA., Kimura B. Levels of angiotensin and molecular biology of the tissue rennin angiotensin systems. Regul Pept. 43:1–20. 1993.Plunkett LM., Correa FM., Saavedra JM. Quantitative autoradiographic determination of angiotensin-converting enzyme binding in rat pituitary and adrenal glands with 124I−351A, a specific inhibitor. Regul Pept. 28:263–272. 1985.Powis DA., O'Brien KJ. Angiotensin II increases catecholamine release from bovine adrenal medulla but does not enhance that evoked by K+ depolarization or by carbachol. J Neurochem. 57:1461–1469. 1991.Saavedra JM. Brain and pituitary angiotensin. Endocr Rev. 13:329–380. 1992.
ArticleSaiki Y., Watanabe T., Tan N., Matsuzaki M., Nakamura S. Role of central ANG-II receptors in stress-induced cardiovascular and hyperthermic responses in rats. Am J Physiol. 272:26–33. 1997.Seidler NW., Jona I., Vegh N., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasimc reticulum. J Biol Chem. 264:17816–17823. 1989.Seltzer A., Bregonzio C., Armando I., Baiardi G., Saavedra JM. Oral administration of an AT1 receptor antagonist prevents the central effects of angiotensin II in spontaneously hypertensive rats. Brain Res. 1028:9–18. 2004.
ArticleStoehr SJ., Smolen JE., Holz RW., Agranoff BW. Inositol trisphosphate mobilizes intracellular calcium in permeabilized adrenal chromaffin cells. J Neurochem. 46:637–640. 1986.
ArticleSuzuki M., Muraki K., Imaizumi Y., Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca2+-pump, reduces Ca2+-dependent K+ currents in guinea-pig smooth muscle cells. Br J Pharmacol. 107:134–140. 1992.Swope SL., Moss SJ., Blackstone CD., Huganir RL. Phosphorylation of ligand-gated ion channels: a possible mode of synaptic plasticity. FASEB J. 6:2514–2523. 1992.
ArticleTakekoshi K., Ishii K., Kawakami Y., Isobe K., Nakai T. Activation of angiotensin II subtype 2 receptor induces catecholamine release in an extracellular Ca2+-dependent manner through a decrease of cyclic guanosine 3′,5′-monophosphate production in cultured porcine adrenal medullary chromaffin cells. Endocrinol. 142:3075–3086. 2001.Tallarida RJ., Murray RB. Manual of pharmacologic calculation with computer programs. 2nd ed.Speringer-Verlag;New York: p. p. 132. 1987.Teschemacher AG., Seward EP. Bidirectional modulation of exocytosis by angiotensin II involves multiple G-protein-regulated transduction pathways in chromaffin cells. The J Neurosci. 20:4776–4785. 2000.
ArticleTimmermans PB., Wong PC., Chiu AT., Herblin WF., Smith RD. New perspectives in angiotensin system control. J Hum Hypertens. 7:19–31. 1993.Uresin Y., Erbas B., Ozek M., Ozkök E., Gürol AO. Losartan may prevent the elevation of plasma glucose, corticosterone and catecholamine levels induced by chronic stress. J Renin Angiotensin Aldosterone Syst. 5:93–96. 2004.
ArticleVijayapandi P., Nagappa AN. Biphasic effects of losartan potassium on immobility in mice. Yakugaku Zasshi. 125:653–657. 2005.
ArticleWada A. Takara H., Izumi F., Kobayashi H., Yanagihara N. Influx of 22Na through acetylcholine receptor-associated Na channels: relationship between 22Na influx, 45Ca influx and secretion of catecholamines in cultured bovine adrenal medulla cells. Neuroscience. 15:283–292. 1985.Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 313:463–480. 1981.
ArticleWong PC., Hart SD., Zaspel AM., Chiu AT., Ardecky RJ., Smith RD., Timmermans PB. Functional studies of nonpeptide angiotensin II receptor subtype-specific ligands: DuP 753 (AII-1) and PD123177 (AII-2). J Pharmacol Exp Ther. 255:584–592. 1990.Worck RH., Frandsen E., Ibsen H., Petersen JS. AT1 and AT2 receptor blockade and epinephrine release during insulin-induced hypoglycemia. Hyperten. 31:384–390. 1998.Yang G., Xi Z., Wan Y., Wang H., Bi G. Changes in circulating and tissue angiotensin II during acute and chronic stress. Biol Signals. 2:166–172. 1993.
ArticleZaman MA., Oparil S., Calhoun DA. Drugs targeting the renin-angiotensin-aldosterone system. Nat Rev Drug Discov. 1:621–636. 2002.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Inhibitory Effects between Enalapril and Losartan on Adrenal Catecholamine Secretion
- Mechanism of epibatidine-induced catecholamine secretion in the rat adrenal gland
- Influence of GABAergic Receptors on Catecholamine Secretion in the Isolated Rat Adrenal Glands
- D-Amphetamine Causes Dual Actions on Catecholamine Release from the Rat Adrenal Medulla
- Effect of Doxorubicin on Catecholamine Release in the Isolated Perfused Rat Adrenal Gland