J Vet Sci.
2014 Dec;15(4):503-509. 10.4142/jvs.2014.15.4.503.
Use of hydrophilic extra-viral domain of canine distemper virus H protein for enzyme-linked immunosorbent assay development
- Affiliations
-
- 1Import Risk Assessment Division, Animal and Plant Quarantine Agency, Anyang 430-757, Korea.
- 2ISU Abxis Co., Ltd. Yonsei University Medical Center, Seoul 120-752, Korea.
- 3Department of Infectious Diseases, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea.
- 4Veterinary Science Research Institute, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea. virus@konkuk.ac.kr
- KMID: 2070236
- DOI: http://doi.org/10.4142/jvs.2014.15.4.503
Abstract
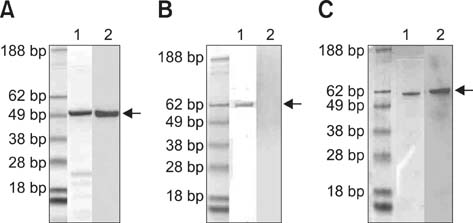
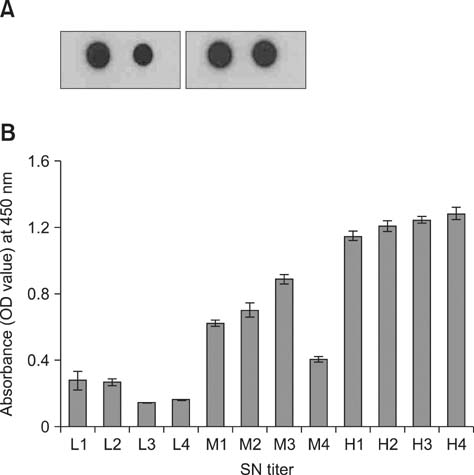
- Simple methods for measuring the levels of serum antibody against canine distemper virus (CDV) would assist in the effective vaccination of dogs. To develop an enzyme-linked immunosorbent assay (ELISA) specific for CDV, we expressed hydrophilic extra-viral domain (HEVD) protein of the A75/17-CDV H gene in a pET 28a plasmid-based Escherichia (E.) coli vector system. Expression was confirmed by dot and Western blotting. We proposed that detection of E. coli-expressed H protein might be conformation-dependent because intensities of the reactions observed with these two methods varied. The H gene HEVD protein was further purified and used as an antigen for an ELISA. Samples from dogs with undetectable to high anti-CDV antibody titers were analyzed using this HEVD-specific ELISA and a commercial CDV antibody detection kit (ImmunoComb). Levels of HEVD antigenicity measured with the assays and immunochromatography correlated. These data indicated that the HEDV protein may be used as antigen to develop techniques for detecting antibodies against CDV.
Keyword
MeSH Terms
-
Animals
Antigens, Viral/*diagnostic use
Distemper/diagnosis/*virology
Distemper Virus, Canine/*immunology
Dog Diseases/*diagnosis/virology
Dogs
Enzyme-Linked Immunosorbent Assay/*veterinary
Escherichia coli/genetics
Genetic Vectors/genetics
Hemagglutinins, Viral/*diagnostic use
Antigens, Viral
Hemagglutinins, Viral
Figure
Reference
-
1. Cha SY, Kim EJ, Kang M, Jang SH, Lee HB, Jang HK. Epidemiology of canine distemper virus in wild raccoon dogs (Nyctereutes procyonoides) from South Korea. Comp Immunol Microbiol Infect Dis. 2012; 35:497–504.
Article2. Chappuis G. Neonatal immunity and immunisation in early age: lessons from veterinary medicine. Vaccine. 1998; 16:1468–1472.
Article3. Cherpillod P, Beck K, Zurbriggen A, Wittek R. Sequence analysis and expression of the attachment and fusion proteins of canine distemper virus wild-type strain A75/17. J Virol. 1999; 73:2263–2269.
Article4. Coyne MJ, Burr JH, Yule TD, Harding MJ, Tresnan DB, McGavin D. Duration of immunity in dogs after vaccination or naturally acquired infection. Vet Rec. 2001; 149:509–515.
Article5. Gemma T, Iwatsuki K, Shin YS, Yoshida E, Kai C, Mikami T. Serological analysis of canine distemper virus using an immunocapture ELISA. J Vet Med Sci. 1996; 58:791–794.
Article6. Iwatsuki K, Miyashita N, Yoshida E, Gemma T, Shin YS, Mori T, Hirayama N, Kai C, Mikami T. Molecular and phylogenetic analyses of the haemagglutinin (H) proteins of field isolates of canine distemper virus from naturally infected dogs. J Gen Virol. 1997; 78:373–380.
Article7. Jung MJ, Moon YC, Cho IH, Yeh JY, Kim SE, Chang WS, Park SY, Song CS, Kim HY, Park KK, McOrist S, Choi IS, Lee JB. Induction of castration by immunization of male dogs with recombinant gonadotropin-releasing hormone (GnRH)-canine distemper virus (CDV) T helper cell epitope p35. J Vet Sci. 2005; 6:21–24.
Article8. Lamb RA. Paramyxovirus fusion: a hypothesis for changes. Virology. 1993; 197:1–11.
Article9. Mochizuki M, Hashimoto M, Hagiwara S, Yoshida Y, Ishiguro S. Genotypes of canine distemper virus determined by analysis of the hemagglutinin genes of recent isolates from dogs in Japan. J Clin Microbiol. 1999; 37:2936–2942.
Article10. Oh JS, Ha GW, Cho YS, Kim MJ, An DJ, Hwang KK, Lim YK, Park BK, Kang B, Song DS. One-step immunochromatography assay kit for detecting antibodies to canine parvovirus. Clin Vaccine Immunol. 2006; 13:520–524.
Article11. Olson P, Klingeborn B, Hedhammar A. Serum antibody response to canine parvovirus, canine adenovirus-1, and canine distemper virus in dogs with known status of immunization: study of dogs in Sweden. Am J Vet Res. 1988; 49:1460–1466.12. Plattet P, Rivals JP, Zuber B, Brunner JM, Zurbriggen A, Wittek R. The fusion protein of wild-type canine distemper virus is a major determinant of persistent infection. Virology. 2005; 337:312–326.
Article13. Rivals JP, Plattet P, Currat-Zweifel C, Zurbriggen A, Wittek R. Adaptation of canine distemper virus to canine footpad keratinocytes modifies polymerase activity and fusogenicity through amino acid substitutions in the P/V/C and H proteins. Virology. 2007; 359:6–18.
Article14. Sidhu MS, Husar W, Cook SD, Dowling PC, Udem SA. Canine distemper terminal and intergenic non-protein coding nucleotide sequences: completion of the entire CDV genome sequence. Virology. 1993; 193:66–72.
Article15. von Messling V, Harder TC, Moennig V, Rautenberg P, Nolte I, Haas L. Rapid and sensitive detection of immunoglobulin M (IgM) and IgG antibodies against canine distemper virus by a new recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay. J Clin Microbiol. 1999; 37:1049–1056.
Article16. von Messling V, Zimmer G, Herrler G, Haas L, Cattaneo R. The hemagglutinin of canine distemper virus determines tropism and cytopathogenicity. J Virol. 2001; 75:6418–6427.
Article17. Waner T, Mazar S, Nachmias E, Keren-Kornblatt E, Harrus S. Evaluation of a dot ELISA kit for measuring immunoglobulin M antibodies to canine parvovirus and distemper virus. Vet Rec. 2003; 152:588–591.
Article18. Winters WD. Time dependent decreases of maternal canine virus antibodies in newborn pups. Vet Rec. 1981; 108:295–299.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incidence of canine viral diseases and prevalence of virus neutralization antibodies of canine distemper virus, adenovirus type 2, parvovirus, and parainfluenza virus type 5 in Korean dogs
- Evaluation of Commercial Immunochromatographic Test Kits for the Detection of Canine Distemper Virus
- Development of an Indirect ELISA Featuring Plates Coated with Column Chromatographically Purified Canine Adenovirus Type-1 Antigen
- Detection of anti-borrelia burgdorferi antibody by enzyme-linked immunosorbent assay in Korea
- Detection of viral infections in wild Korean raccoon dogs (Nyctereutes procyonoides koreensis)