Yonsei Med J.
2014 Nov;55(6):1473-1483. 10.3349/ymj.2014.55.6.1473.
Immunologic Evaluation of Immediate Hypersensitivity to Cefaclor
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. hspark@ajou.ac.kr
- 2Division of Allergy and Clinical Immunology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2070193
- DOI: http://doi.org/10.3349/ymj.2014.55.6.1473
Abstract
- PURPOSE
Cefaclor is widely prescribed for various infectious diseases. As its consumption increases, the number of hypersensitivity reactions to cefaclor has increased. This study aimed to evaluate the immunologic findings of immediate hypersensitivity to cefaclor.
MATERIALS AND METHODS
We enrolled 47 patients with immediate hypersensitivity to cefaclor from Ajou University Hospital and Asan Medical Center. Serum specific IgE, IgG1, and IgG4 antibodies to cefaclor-human serum albumin (HSA) conjugate were measured by enzyme-linked immunosorbent assay (ELISA).
RESULTS
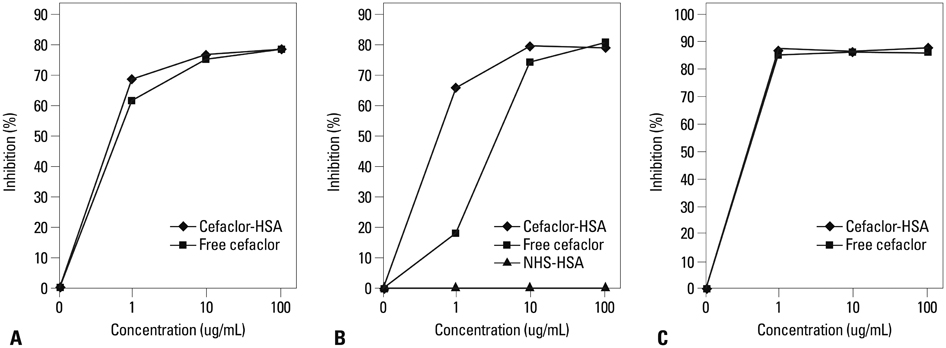
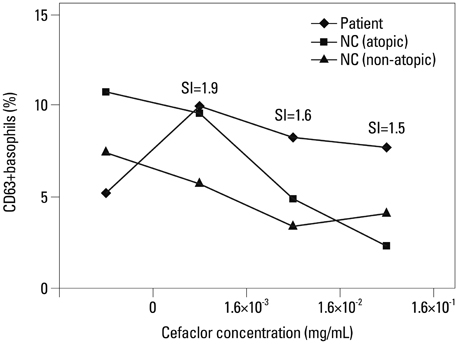
The most common phenotype was anaphylaxis (Group I, 78.7%), followed by urticaria (Group II, 21.3%). The detection of specific IgE, IgG1, and IgG4 to cefaclor-HSA conjugate by ELISA tended to be higher in Group I (40.5%, 41.7%, 21.6%) than in Group II (20.0%, 20.0%, 0%) with no statistical significance. Significant associations were found between specific IgE and IgG1 or IgG4 (p<0.001, p=0.019). ELISA inhibition tests showed significant inhibitions by both free cefaclor and cefaclor-HSA conjugate. For basophil activation tests in patients having no specific IgE antibody, the CD63 expression level on basophils increased with incubations of free cefaclor.
CONCLUSION
The most common manifestation of immediate hypersensitivity to cefaclor was anaphylaxis, most of which was mediated by IgE; however, a non-IgE mediated direct basophil activation mechanism was suggested in a subset of anaphylaxis patients.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Anaphylaxis/*chemically induced/immunology
Anti-Bacterial Agents/adverse effects/*immunology
Antigens, CD63
Basophils/metabolism
Cefaclor/*adverse effects/immunology
Enzyme-Linked Immunosorbent Assay
Female
Humans
Hypersensitivity, Immediate/chemically induced/diagnosis/*immunology
Immunoglobulin E/*blood
Immunoglobulin G/immunology
Male
Middle Aged
Retrospective Studies
Skin Tests
Urticaria/chemically induced/diagnosis/immunology
Young Adult
Anti-Bacterial Agents
Antigens, CD63
Cefaclor
Immunoglobulin E
Immunoglobulin G
Figure
Cited by 1 articles
-
Proper Cut-off Levels of Serum Specific IgE to Cefaclor for Patients with Cefaclor Allergy
Young-Hee Nam, So-Hee Lee, Hyo-In Rhyou, Young-Soo Lee, Seung-Hee Park, Young-Hee Lee, Yoo-Seob Shin, Hae-Sim Park, Young-Min Ye
Yonsei Med J. 2018;59(8):968-974. doi: 10.3349/ymj.2018.59.8.968.
Reference
-
1. Moreno E, Macías E, Dávila I, Laffond E, Ruiz A, Lorente F. Hypersensitivity reactions to cephalosporins. Expert Opin Drug Saf. 2008; 7:295–304.
Article2. Kim JE, Kim SH, Jin HJ, Hwang EK, Kim JH, Ye YM, et al. IgE sensitization to cephalosporins in health care workers. Allergy Asthma Immunol Res. 2012; 4:85–91.
Article3. Dickson SD, Salazar KC. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol. 2013; 45:131–142.
Article4. Blanca M, Romano A, Torres MJ, Férnandez J, Mayorga C, Rodriguez J, et al. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009; 64:183–193.
Article5. Grouhi M, Hummel D, Roifman CM. Anaphylactic reaction to oral cefaclor in a child. Pediatrics. 1999; 103:e50.
Article6. Atanasković-Marković M, Velicković TC, Gavrović-Jankulović M, Vucković O, Nestorović B. Immediate allergic reactions to cephalosporins and penicillins and their cross-reactivity in children. Pediatr Allergy Immunol. 2005; 16:341–347.
Article7. Gómez E, Torres MJ, Mayorga C, Blanca M. Immunologic evaluation of drug allergy. Allergy Asthma Immunol Res. 2012; 4:251–263.
Article8. Nam YH, Kim JE, Hwang EK, Jin HJ, Shin YS, Ye YM, et al. Clinical and immunologic evaluations of immediate hypersensitivity to cefaclor. Korean J Asthma Allergy Clin Immunol. 2011; 31:192–198.9. Ben-Shoshan M, Clarke AE. Anaphylaxis: past, present and future. Allergy. 2011; 66:1–14.
Article10. Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004; 114:371–376.
Article11. Kim SH, Choi JH, Park HS. Heterogeneity of the IgE response to allergenic determinants of cefaclor in serum samples from patients with cefaclor-induced anaphylaxis. Ann Allergy Asthma Immunol. 2005; 94:700–704.
Article12. Nam YH, Kim JE, Kim SH, Jin HJ, Hwang EK, Shin YS, et al. Identifying genetic susceptibility to sensitization to cephalosporins in health care workers. J Korean Med Sci. 2012; 27:1292–1299.
Article13. Kim JH, An S, Kim JE, Choi GS, Ye YM, Park HS. Beef-induced anaphylaxis confirmed by the basophil activation test. Allergy Asthma Immunol Res. 2010; 2:206–208.
Article14. Kim MS, Cho YJ. Flow cytometry-assisted basophil activation test as a safe diagnostic tool for Aspirin/NSAID hypersenstivity. Allergy Asthma Immunol Res. 2012; 4:137–142.
Article15. Gruchalla RS, Pirmohamed M. Clinical practice. Antibiotic allergy. N Engl J Med. 2006; 354:601–609.16. Sohn HS, Oh OH, Kwon JW, Lee YS. Higher systemic antibiotic consumption in a population of South Korea (2008-2009). Int J Clin Pharmacol Ther. 2013; 51:585–592.
Article17. Novembre E, Mori F, Pucci N, Bernardini R, Romano A. Cefaclor anaphylaxis in children. Allergy. 2009; 64:1233–1235.
Article18. Beghetti M, Wilson GJ, Bohn D, Benson L. Hypersensitivity myocarditis caused by an allergic reaction to cefaclor. J Pediatr. 1998; 132:172–173.
Article19. Romano A, Gaeta F, Valluzzi RL, Alonzi C, Viola M, Bousquet PJ. Diagnosing hypersensitivity reactions to cephalosporins in children. Pediatrics. 2008; 122:521–527.
Article20. Nishioka K, Katayama I, Kobayashi Y, Takijiri C. Anaphylaxis due to cefaclor hypersensitivity. J Dermatol. 1986; 13:226–227.
Article21. Norrby SR. Side effects of cephalosporins. Drugs. 1987; 34:Suppl 2. 105–120.
Article22. Annè S, Reisman RE. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Ann Allergy Asthma Immunol. 1995; 74:167–170.23. Hama R, Mori K. High incidence of anaphylactic reactions to cefaclor. Lancet. 1988; 1:1331.
Article24. Kammer RB. Cefaclor in management of streptococcal pharyngitis, otitis media, and skin infections. Ann Otol Rhinol Laryngol Suppl. 1981; 90(3 Pt 3):79–81.
Article25. Patel DA, Holdford DA, Edwards E, Carroll NV. Estimating the economic burden of food-induced allergic reactions and anaphylaxis in the United States. J Allergy Clin Immunol. 2011; 128:110–115.
Article26. Mullins RJ. Anaphylaxis: risk factors for recurrence. Clin Exp Allergy. 2003; 33:1033–1040.
Article27. Joint Task Force on Practice Parameters. American Academy of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology. Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010; 105:259–273.28. Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968; 38:159–188.29. Kim MJ, Choi GS, Um SJ, Sung JM, Shin YS, Park HJ, et al. Anaphylaxis; 10 years' experience at a university hospital in Suwon. Korean J Asthma Allergy Clin Immunol. 2008; 28:298–304.30. Somech R, Weber EA, Lavi S. Evaluation of immediate allergic reactions to cephalosporins in non-penicillin-allergic patients. Int Arch Allergy Immunol. 2009; 150:205–209.
Article31. Yoon SY, Park SY, Kim S, Lee T, Lee YS, Kwon HS, et al. Validation of the cephalosporin intradermal skin test for predicting immediate hypersensitivity: a prospective study with drug challenge. Allergy. 2013; 68:938–944.
Article32. Atanasković-Marković M, Gavrović-Jankulović M, Cirković Velicković T, Vucković O, Todorić D. Type-I hypersensitivity to ceftriaxone and cross-reactivity with cefalexin and ampicillin. Allergy. 2003; 58:537–538.
Article33. Pham NH, Baldo BA. beta-Lactam drug allergens: fine structural recognition patterns of cephalosporin-reactive IgE antibodies. J Mol Recognit. 1996; 9:287–296.
Article34. Jiao D, Liu Y, Lu X, Pan Q, Zheng J, Liu B, et al. Characteristics of anaphylaxis-inducing IgG immune complexes triggering murine passive systemic anaphylaxis. Allergy. 2013; 68:236–245.
Article35. Dash CH. Penicillin allergy and the cephalosporins. J Antimicrob Chemother. 1975; 1:3 Suppl. 107–118.
Article36. Saxon A, Beall GN, Rohr AS, Adelman DC. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med. 1987; 107:204–215.
Article37. Petz LD. Immunologic cross-reactivity between penicillins and cephalosporins: a review. J Infect Dis. 1978; 137:Suppl. S74–S79.
Article38. Petz LD. Immunologic reactions of humans to cephalosporins. Postgrad Med J. 1971; 47:Suppl. 64–69.39. Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010; 125:2 Suppl 2. S126–S137.
Article40. Romano A, Guéant-Rodriguez RM, Viola M, Pettinato R, Guéant JL. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med. 2004; 141:16–22.
Article41. Macy E, Mangat R, Burchette RJ. Penicillin skin testing in advance of need: multiyear follow-up in 568 test result-negative subjects exposed to oral penicillins. J Allergy Clin Immunol. 2003; 111:1111–1115.
Article42. Antúnez C, Fernández T, Blanca-Lopez N, Torres MJ, Mayorga C, Canto G, et al. IgE antibodies to betalactams: relationship between the triggering hapten and the specificity of the immune response. Allergy. 2006; 61:940–946.
Article43. Audicana M, Bernaola G, Urrutia I, Echechipia S, Gastaminza G, Muñoz D, et al. Allergic reactions to betalactams: studies in a group of patients allergic to penicillin and evaluation of cross-reactivity with cephalosporin. Allergy. 1994; 49:108–113.
Article44. Blanca M, Fernandez J, Miranda A, Terrados S, Torres MJ, Vega JM, et al. Cross-reactivity between penicillins and cephalosporins: clinical and immunologic studies. J Allergy Clin Immunol. 1989; 83(2 Pt 1):381–385.
Article45. Katsutani N, Shionoya H. Immunogenicity of various beta-lactam antibiotic-protein conjugates and cross-reactivity of the antibodies produced in guinea pig. Int Arch Allergy Immunol. 1993; 100:128–134.
Article46. Miranda A, Blanca M, Vega JM, Moreno F, Carmona MJ, García JJ, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol. 1996; 98:671–677.
Article47. Sastre J, Quijano LD, Novalbos A, Hernandez G, Cuesta J, de las Heras M, et al. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy. 1996; 51:383–386.
Article48. Jönsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, et al. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011; 121:1484–1496.
Article49. Posadas SJ, Pichler WJ. Delayed drug hypersensitivity reactions - new concepts. Clin Exp Allergy. 2007; 37:989–999.
Article50. Ishizaka A, Sakiyama Y, Nakanishi M, Tomizawa K, Oshika E, Kojima K, et al. The inductive effect of interleukin-4 on IgG4 and IgE synthesis in human peripheral blood lymphocytes. Clin Exp Immunol. 1990; 79:392–396.
Article51. De Bisschop MB, Bellou A. Anaphylaxis. Curr Opin Crit Care. 2012; 18:308–317.
Article52. Blunk JA, Schmelz M, Zeck S, Skov P, Likar R, Koppert W. Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: an in vivo microdialysis study in human skin. Anesth Analg. 2004; 98:364–370.53. Simon RA, Schatz M, Stevenson DD, Curry N, Yamamoto F, Plow E, et al. Radiographic contrast media infusions. Measurement of histamine, complement, and fibrin split products and correlation with clinical parameters. J Allergy Clin Immunol. 1979; 63:281–288.54. Ring J, Simon RA, Arroyave CM. Increased in vitro histamine release by radiographic contrast media in patients with history of incompatibility. Clin Exp Immunol. 1978; 34:302–309.55. Simons FE, Ardusso LR, Bilò MB, El-Gamal YM, Ledford DK, Ring J, et al. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011; 4:13–37.
Article56. Pumphrey RS, Davis S. Under-reporting of antibiotic anaphylaxis may put patients at risk. Lancet. 1999; 353:1157–1158.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Anaphylaxis after Exposure to Oral Cefaclor
- Clinical and Immunologic Evaluations of Immediate Hypersensitivity to Cefaclor
- Proper Cut-off Levels of Serum Specific IgE to Cefaclor for Patients with Cefaclor Allergy
- A case of immediate hypersensitivity to cefaclor: serum specific IgE detection
- A Case of Anaphylaxis Induced by Oral Cefaclor in Aspirin Idiosyncratic Patient