Korean J Radiol.
2015 Feb;16(1):133-138. 10.3348/kjr.2015.16.1.133.
Evaluation of Arterial Impairment after Experimental Gelatin Sponge Embolization in a Rabbit Renal Model
- Affiliations
-
- 1Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea. hgleehfh@catholic.ac.kr
- 2Department of Hospital Pathology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- KMID: 2069991
- DOI: http://doi.org/10.3348/kjr.2015.16.1.133
Abstract
OBJECTIVE
Arterial stenosis is a major obstacle for subsequent interventional procedures. We hypothesized that the stenosis is caused by gelatin sponge embolization and performed an experimental study in a rabbit renal model.
MATERIALS AND METHODS
A total of 24 rabbits were embolized with porcine gelatin sponge particles injected into the renal arteries. Four rabbits were sacrificed on 1 day, 4 days, 1 week, 2 weeks, 3 weeks, and 4 weeks after embolization. Microscopic evaluations were performed on hematoxylin-eosin and smooth muscle actin immunohistochemical stained sections.
RESULTS
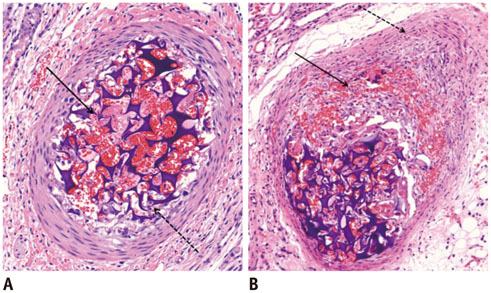
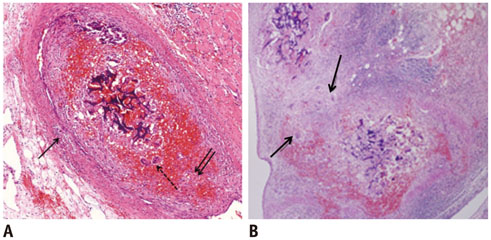
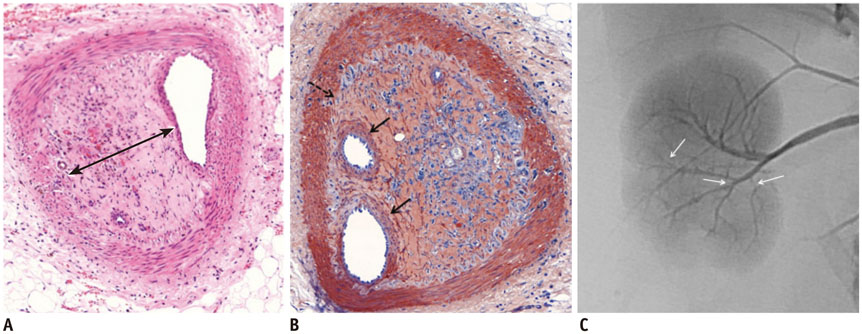
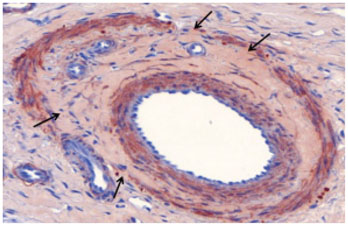
Gelatin sponge particles were mainly observed in the segmental and interlobar arteries. Transmural inflammation of the embolized arterial wall and mild thickening of the media were observed 1 week after embolization. Resorption of the gelatin sponge and organization of thrombus accompanied by foreign body reactions, were observed from 2 to 4 weeks after embolization. Microscopic images of the 3 weeks group showed vessel lumens filled mostly with organized thrombi, resulting in severe stenosis. Additionally, vessels showed a thickened intima that contained migrating smooth muscle cells and accompanying interruption of the internal elastic lamina. The migrating smooth muscle cells were distributed around the recanalized arterial lumen.
CONCLUSION
Gelatin sponge embolization may induce arterial stenosis by causing organized thrombus and intimal hyperplasia, which consists of migrating smooth muscle cells and intimal collagen deposits.
Keyword
MeSH Terms
Figure
Reference
-
1. Doyon D, Mouzon A, Jourde AM, Regensberg C, Frileux C. [Hepatic, arterial embolization in patients with malignant liver tumours (author's transl)]. Ann Radiol (Paris). 1974; 17:593–603.2. Goldstein HM, Wallace S, Anderson JH, Bree RL, Gianturco C. Transcatheter occlusion of abdominal tumors. Radiology. 1976; 120:539–545.3. Katsumori T, Kasahara T. The size of gelatin sponge particles: differences with preparation method. Cardiovasc Intervent Radiol. 2006; 29:1077–1083.4. Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983; 148:397–401.5. Erinjeri JP, Salhab HM, Covey AM, Getrajdman GI, Brown KT. Arterial patency after repeated hepatic artery bland particle embolization. J Vasc Interv Radiol. 2010; 21:522–526.6. Sueyoshi E, Hayashida T, Sakamoto I, Uetani M. Vascular complications of hepatic artery after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2010; 195:245–251.7. Maeda N, Osuga K, Mikami K, Higashihara H, Onishi H, Nakaya Y, et al. Angiographic evaluation of hepatic arterial damage after transarterial chemoembolization for hepatocellular carcinoma. Radiat Med. 2008; 26:206–212.8. Sahara S, Kawai N, Sato M, Tanaka T, Ikoma A, Nakata K, et al. Prospective evaluation of transcatheter arterial chemoembolization (TACE) with multiple anti-cancer drugs (epirubicin, cisplatin, mitomycin c, 5-fluorouracil) compared with TACE with epirubicin for treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2012; 35:1363–1371.9. Brown DB, Pilgram TK, Darcy MD, Fundakowski CE, Lisker-Melman M, Chapman WC, et al. Hepatic arterial chemoembolization for hepatocellular carcinoma: comparison of survival rates with different embolic agents. J Vasc Interv Radiol. 2005; 16:1661–1666.10. Katsumori T, Nakajima K, Mihara T, Tokuhiro M. Uterine artery embolization using gelatin sponge particles alone for symptomatic uterine fibroids: midterm results. AJR Am J Roentgenol. 2002; 178:135–139.11. Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. Br J Surg. 1994; 81:1254–1269.12. Clowes AW. Intimal hyperplasia and graft failure. Cardiovasc Pathol. 1993; 2:179S–186S.13. Liu MW, Roubin GS, King SB 3rd. Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989; 79:1374–1387.14. Carrel A, Guthrie CC. Anastomosis of blood vessels by the patching method and transplantation of the kidney. 1906 [classical article]. Yale J Biol Med. 2001; 74:243–247.15. Waltham M, Harris J. Intimal hyperplasia: the nemesis of cardiovascular intervention. ANZ J Surg. 2004; 74:719–720.16. Strauss BH, Wilson RA, van Houten R, van Suylen RJ, Murphy ES, Escaned J, et al. Late effects of locally delivered mitomycin C on formation of neointima and on vasomotor response to acetylcholine. Coron Artery Dis. 1994; 5:633–641.17. Richard HM 3rd, Silberzweig JE, Mitty HA, Lou WY, Ahn J, Cooper JM. Hepatic arterial complications in liver transplant recipients treated with pretransplantation chemoembolization for hepatocellular carcinoma. Radiology. 2000; 214:775–779.18. Siskin GP, Dowling K, Virmani R, Jones R, Todd D. Pathologic evaluation of a spherical polyvinyl alcohol embolic agent in a porcine renal model. J Vasc Interv Radiol. 2003; 14:89–98.19. Stampfl S, Bellemann N, Stampfl U, Radeleff B, Lopez-Benitez R, Sommer CM, et al. Inflammation and recanalization of four different spherical embolization agents in the porcine kidney model. J Vasc Interv Radiol. 2008; 19:577–586.20. Kawai N, Sato M, Minamiguchi H, Ikoma A, Sanda H, Nakata K, et al. Clinical evaluation of transcatheter arterial chemoembolization with 2-day-soluble gelatin sponge particles for hepatocellular carcinoma-comparison with insoluble gelatin sponge particles. J Vasc Interv Radiol. 2013; 24:1383–1390.21. Takasaka I, Kawai N, Sato M, Sahara S, Minamiguchi H, Nakai M, et al. A new soluble gelatin sponge for transcatheter hepatic arterial embolization. Cardiovasc Intervent Radiol. 2010; 33:1198–1204.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transcatheter arterial embolization for congenital renal arteriovenous fistula
- Gelatin Sponge Particle Embolization of Spontaneously Ruptured Intrahepatic Arterial Aneurysms in a Patient with Polyarteritis Nodosa: A Case Report
- An experimental microangiographic study on renal embolization with various embolic materials
- Degradable Gelatin Microspheres as an Embolic Agent: an Experimental Study in a Rabbit Renal Model
- A Case of Control of Renal Hemorrhage by Selective Renal Arterial Embolization