Korean J Radiol.
2007 Oct;8(5):418-428. 10.3348/kjr.2007.8.5.418.
Degradable Gelatin Microspheres as an Embolic Agent: an Experimental Study in a Rabbit Renal Model
- Affiliations
-
- 1Department of Radiology, Shiga University of Medical Science, Shiga, Japan. junryuhei@belle.shiga-med.ac.jp
- 2Institute for Frontier Medical Sciences, Kyoto University, Kyoto, Japan.
- KMID: 1734291
- DOI: http://doi.org/10.3348/kjr.2007.8.5.418
Abstract
OBJECTIVE
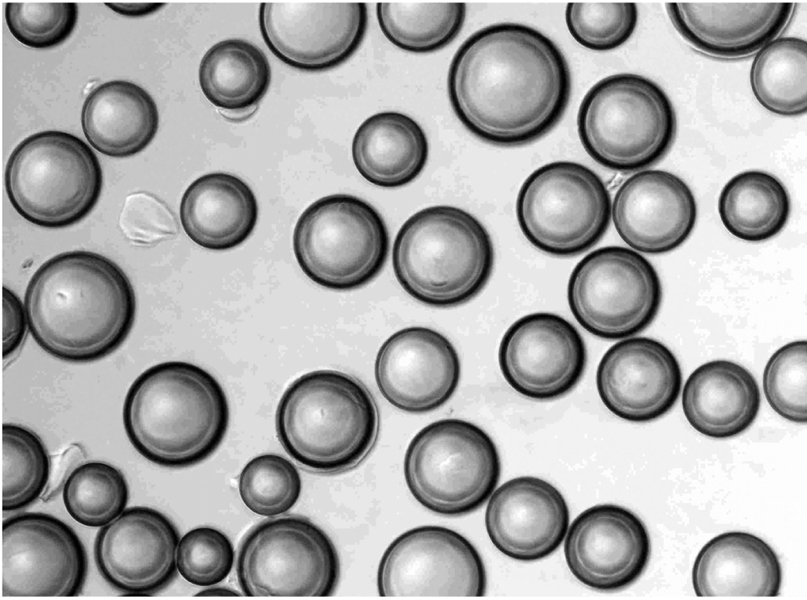
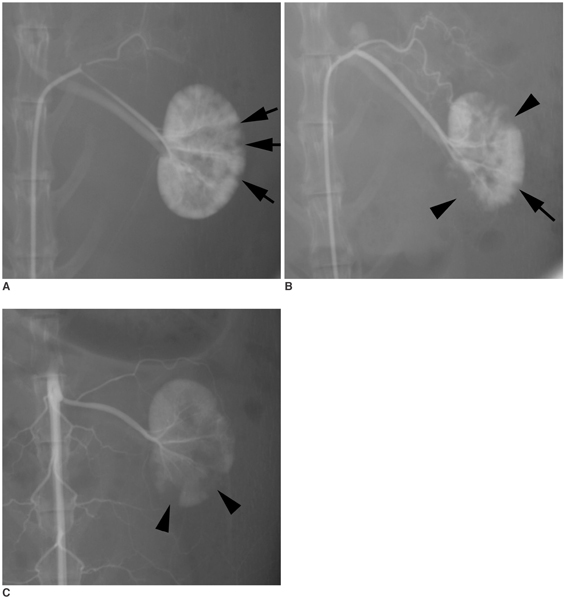
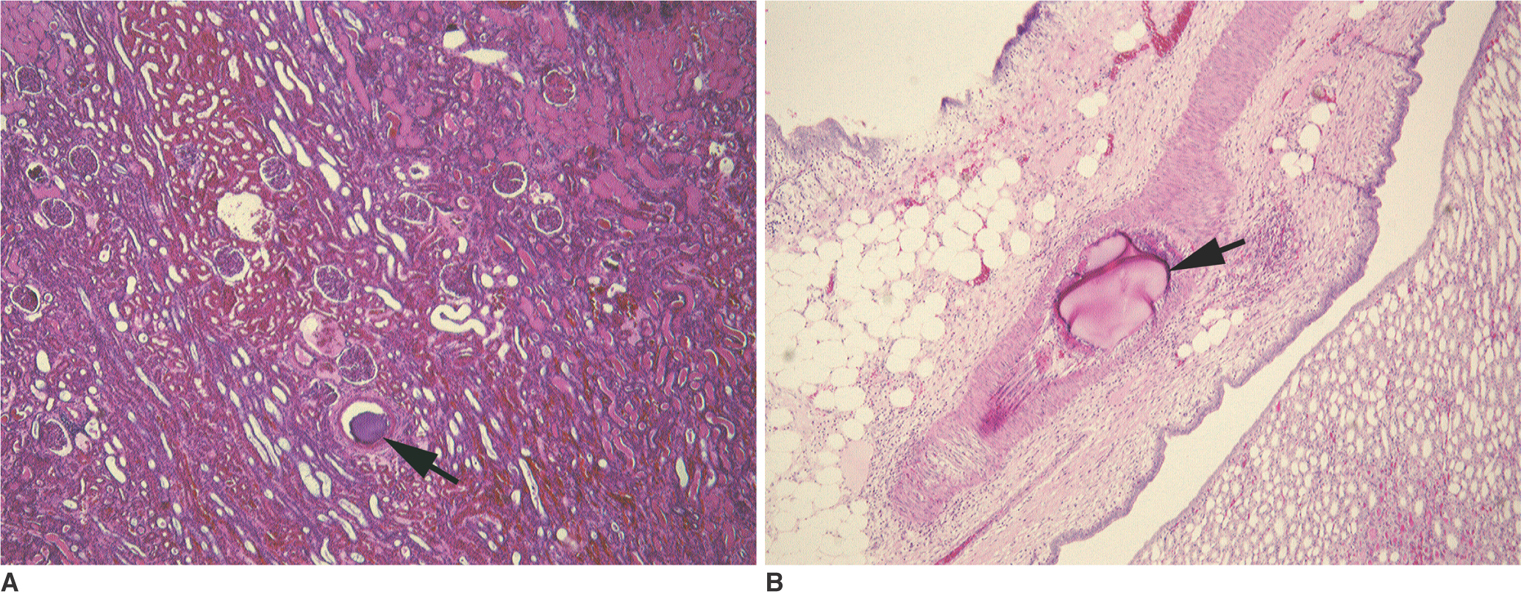
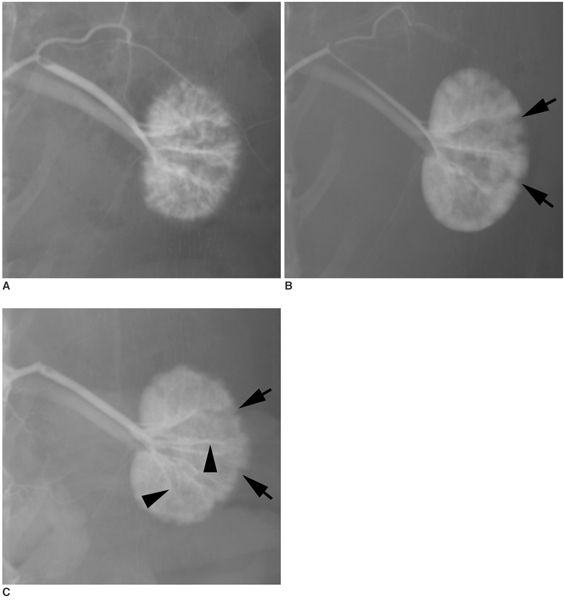
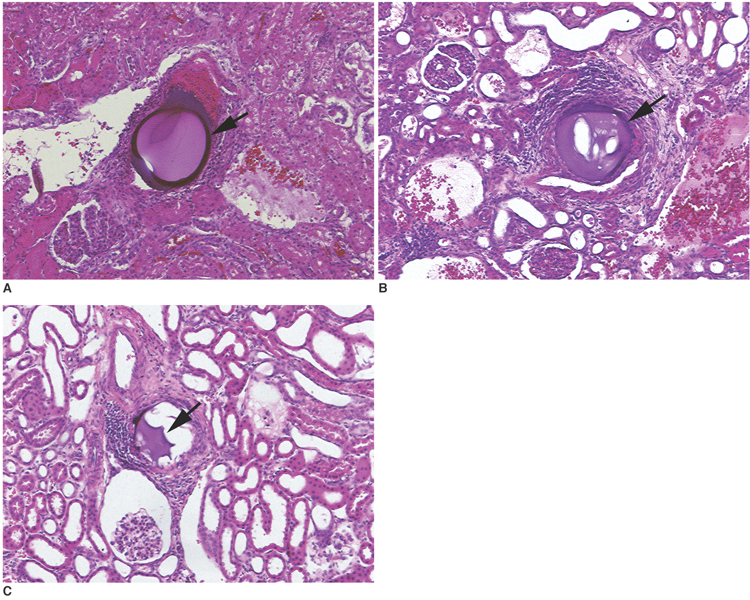
To investigate the basic characteristics of degradable gelatin microspheres (GMSs), including their embolic behavior and degradation periods when they are used as embolic materials in the renal arteries of rabbit models. MATERIALS AND METHODS: Based on the GMS particle size, 24 kidneys were divided into 3 groups of eight kidneys, and each group was embolized with a different GMS particle size (group 1: 35-100 micrometer, group 2: 100-200 micrometer, and group 3: 200-300 micrometer). From each group, two rabbits were sacrificed immediately after embolization (day 0), and a pair of rabbits from each group underwent an angiogram and were sacrificed on days 3, 7, and 14, respectively, after embolization. The level of arterial occlusion, the pathological changes in the renal parenchyma, and the degradation of the GMSs were evaluated angiographically and histologically. RESULTS: A follow-up angiogram on days 0, 3, 7, and 14 revealed the presence of wedge-shaped poorly-enhanced areas in the parenchymal phase as seen in all groups. The size of these areas tended to increase with the particle diameter, and persisted up to day 14. On days 3, 7, and 14, parenchymal infarctions were observed histologically in all cases, and this observation corresponded with the parenchyma being supplied by the embolized arteries. GMSs of group 1 mainly reached the interlobular arteries, while those of group 3 mainly reached the interlobar arteries. In all but two cases, the GMSs were identified histologically even on day 14, and sequential degradation was histologically identified in all GMS groups. CONCLUSION: GMSs can be used as degradable embolic materials which can control the level of embolization.
Keyword
MeSH Terms
Figure
Reference
-
1. Feldman F, Casarella WJ, Dick HM, Hollander BA. Selective intra-arterial embolization of bone tumors. A useful adjunct in the management of selected lesions. Am J Roentgenol Radium Ther Nucl Med. 1975. 123:130–139.Rosch J., Dotter CT., Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1972. 102:303–306.3. Pugatch RD, Wolpert SM. Transfemoral embolization of an external carotid-cavernous fistula. Case report. J Neurosurg. 1975. 42:94–97.4. Kunstlinger F, Brunelle F, Chaumont P, Doyon D. Vascular occlusive agents. AJR Am J Roentgenol. 1981. 136:151–156.5. Spies JB, Benenati JF, Worthington-Kirsch RL, Pelage JP. Initial experience with use of tris-acryl gelatin microspheres for uterine artery embolization for leiomyomata. J Vasc Interv Radiol. 2001. 12:1059–1063.6. Jiaqi Y, Hori S, Minamitani K, Hashimoto T, Yoshimura H, Nomura N, et al. A new embolic material: super absorbent polymer (SAP) microsphere and its embolic effects. Nippon Igaku Hoshasen Gakkai Zassh. 1996. 56:19–24.7. Coley SC, Jackson JE. Endovascular occlusion with a new mechanical detachable coil. AJR Am J Roentgenol. 1998. 171:1075–1079.8. Katsumori T, Nakajima K, Mihara T, Tokuhiro M. Uterine artery embolization using gelatin sponge particles alone for symptomatic uterine fibroids: midterm results. AJR Am J Roentgenol. 2002. 178:135–139.9. Sniderman KW, Sos TA, Alonso DR. Transcatheter embolization with Gelfoam and Avitene: the effect of Sotradecol on the duration of arterial occlusion. Invest Radiol. 1981. 16:501–507.10. Vlahos L, Benakis V, Dimakakos P, Dimopoulos C, Pontifex G. A comparative study of the degree of arterial recanalization in kidneys of dogs following transcatheter embolization with eight different materials. Eur Urol. 1980. 6:180–185.11. Tabata Y, Uno K, Ikada Y, Muramatsu S. Potentiation of antitumor activity of macrophages by recombinant interferon alpha A/D contained in gelatin microspheres. Jpn J Cancer Res. 1988. 79:636–646.12. Tabata Y, Ikada Y. Synthesis of gelatin microspheres containing interferon. Pharm Res. 1989. 6:422–427.13. Tabata Y, Uno K, Muramatsu S, Ikada Y. In vivo effects of recombinant interferon alpha A/D incorporated in gelatin microspheres on murine tumor cell growth. Jpn J Cancer Res. 1989. 80:387–393.14. Tabata Y, Miyao M, Inamoto T, Ishii T, Hirano Y, Yamaoki Y, et al. De novo formation of adipose tissue by controlled release of basic fibroblast growth factor. Tissue Eng. 2000. 6:279–289.15. Tabata Y, Hong L, Miyamoto S, Miyao M, Hashimoto N, Ikada Y. Bone formation at a rabbit skull defect by autologous bone marrow cells combined with gelatin microspheres containing TGF-beta1. J Biomater Sci Polym Ed. 2000. 11:891–901.16. Kushibiki T, Matsumoto K, Nakamura T, Tabata Y. Suppression of the progress of disseminated pancreatic cancer cells by NK4 plasmid DNA released from cationized gelatin microspheres. Pharm Res. 2004. 21:1109–1118.17. Gelfoam® Sterile Powder. Accessed March 23, 2006. U.S. Food and Drug Administration;Available at http://www.fda.gov/cdrh/pdf/n18286s012.html.18. Correll JT, Prentice HR, Wise EC. Biologic investigations of a new absorbable sponge. Surg Gynecol Obstet. 1945. 81:585–589.19. Ozeki M, Tabata Y. In vivo degradability of hydrogels prepared from different gelatins by various cross-linking methods. J Biomater Sci Polym Ed. 2005. 16:549–561.20. Brown DB, Pilgram TK, Darcy MD, Fundakowski CE, Lisker-Melman M, Chapman WC, et al. Hepatic arterial chemoembolization for hepatocellular carcinoma: comparison of survival rates with different embolic agents. J Vasc Interv Radiol. 2005. 16:1661–1666.21. Jinbo G. Science of Particles. 1985. Tokyo: Kodansya;12–24.22. Theo G.M., van de Ven. Theo G.M., van de Ven, editors. Classical DLVO therapy. Colloidal Hydrodynamics. 1989. San Diego: Academic Press;39–41.23. Goldstein HM, Wallace S, Anderson JH, Bree RL, Gianturco C. Transcatheter occlusion of abdominal tumors. Radiology. 1976. 120:539–545.24. Tryggvason K, Huhtala P, Höyhtya M, Hujanen E, Hurskainen T. 70 K type IV collagenase (gelatinase). Matrix Suppl. 1992. 1:45–50.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Arterial Impairment after Experimental Gelatin Sponge Embolization in a Rabbit Renal Model
- A New Liquid Embolic Agent(Embol) for Transcatheter Renal Artery Embolization: An Experimental Study in Rabbit
- Efficacy of Renal Artery Embolization using a Mixture of Histoacryl(R) and Lipiodol in a Rabbit Model
- Transplantation of Gelatin Microspheres Loaded with Wharton’s Jelly Derived Mesenchymal Stem Cells Facilitates Cartilage Repair in Mice
- An experimental microangiographic study on renal embolization with various embolic materials