Korean J Urol.
2014 Sep;55(9):599-607. 10.4111/kju.2014.55.9.599.
Nonresponders to Daily Paroxetine and Another SSRI in Men With Lifelong Premature Ejaculation: A Pharmacokinetic Dose-Escalation Study for a Rare Phenomenon
- Affiliations
-
- 1Division of Pharmacology, Utrecht Institute for Pharmaceutical Sciences, Faculty of BetaSciences, Utrecht University, Utrecht, The Netherlands. md@waldinger.demon.nl
- 2Department of Central Hospital Pharmacy, Viecuri Hospital, Venlo, The Netherlands.
- 3Apotheek Haagse Ziekenhuizen, HagaZiekenhuis, Den Haag, The Netherlands.
- 4Department of Internal Medicine and Endocrinology, Reinier de Graaf Groep of Hospitals, Delft-Voorburg, The Netherlands.
- 5Department of Neurosexology, HagaZiekenhuis, Den Haag, The Netherlands.
- KMID: 2069778
- DOI: http://doi.org/10.4111/kju.2014.55.9.599
Abstract
- PURPOSE
Nonresponse to any selective serotonin reuptake inhibitor (SSRI) treatment is rare. In this study, we aimed to investigate ejaculation delay nonresponse to paroxetine treatment in men with lifelong premature ejaculation (PE) who were also known to be nonresponders to other SSRIs.
MATERIALS AND METHODS
Five males with lifelong PE who were known nonresponders to paroxetine and other serotonergic antidepressants and eight males with lifelong PE who were specifically recruited were included. Blood sampling occurred 1 month and 1 day before the start of treatment and at the end of three consecutive series of 4 weeks of daily treatment with 10-, 20-, and 30-mg paroxetine, respectively. Blood samples for measurement of leptin and paroxetine were taken at 8:30 AM, 9:30 AM, 10:30 AM, and 11:30 AM, respectively. At 9:00 AM, one tablet of 10-, 20-, or 30-mg paroxetine was taken during the first, second, and third month, respectively. Intravaginal ejaculatory latency time (IELT) was measured with a stopwatch. The main outcome measures were the fold increase in the geometric mean IELT, serum leptin and paroxetine concentrations, body mass index (BMI), 5-HT1A receptor C-1019G polymorphism, and CYP2D6 mutations.
RESULTS
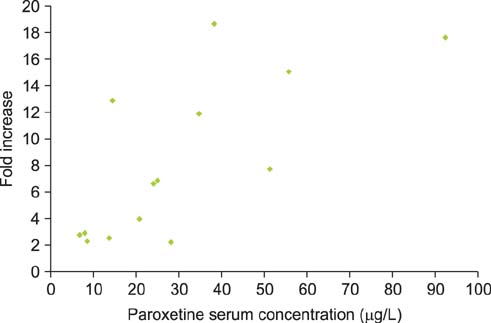
Between the 7 paroxetine responders and 6 nonresponders, the fold increase in the geometric mean IELT was significantly different after daily 10-mg (p=0.003), 20-mg (p=0.002), and 30-mg paroxetine (p=0.026) and ranged from 2.0 to 8.8 and from 1.1 to 1.7, respectively. BMI at baseline and at the end of the study was not significantly different between responders and nonresponders. Serum leptin levels at baseline were similar in responders and nonresponders and did not change during treatment. The serum paroxetine concentration increased with increasing dosage and was not significantly different between responders and nonresponders. There was no association between the fold increase in the geometric mean IELT and serum paroxetine levels during the three treatment periods nor between leptin levels during the treatment periods and serum paroxetine levels. For the 5-HT1A receptor C-1019G variation, all responders had the CC genotype and all nonresponders had the GC genotype, respectively.
CONCLUSIONS
Complete absence of paroxetine-induced ejaculation delay is presumably related to pharmacodynamic factors and perhaps to 5-HT1A receptor gene polymorphism.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Body Mass Index
Cytochrome P-450 CYP2D6/genetics
Humans
Leptin/blood
Male
Middle Aged
Mutation
Paroxetine/*administration & dosage/blood
Polymorphism, Genetic
Premature Ejaculation/*drug therapy/genetics
Receptor, Serotonin, 5-HT1A/genetics
Risk Factors
Serotonin Uptake Inhibitors/*administration & dosage
Time Factors
Treatment Outcome
Young Adult
Cytochrome P-450 CYP2D6
Leptin
Paroxetine
Receptor, Serotonin, 5-HT1A
Serotonin Uptake Inhibitors
Figure
Reference
-
1. Waldinger MD, Zwinderman AH, Schweitzer DH, Olivier B. Relevance of methodological design for the interpretation of efficacy of drug treatment of premature ejaculation: a systematic review and meta-analysis. Int J Impot Res. 2004; 16:369–381.2. Janssen PK, Zwinderman AH, Olivier B, Waldinger MD. Serotonin transporter promoter region (5-HTTLPR) polymorphism is not associated with paroxetine-induced ejaculation delay in Dutch men with lifelong premature ejaculation. Korean J Urol. 2014; 55:129–133.3. Salonia A, Rocchini L, Sacca' A, Pellucchi F, Ferrari M, Carro UD, et al. Acceptance of and discontinuation rate from paroxetine treatment in patients with lifelong premature ejaculation. J Sex Med. 2009; 6:2868–2877.4. Sindrup SH, Brosen K, Gram LF, Hallas J, Skjelbo E, Allen A, et al. The relationship between paroxetine and the sparteine oxidation polymorphism. Clin Pharmacol Ther. 1992; 51:278–287.5. de Jong TR, Pattij T, Veening JG, Waldinger MD, Cools AR, Olivier B. Effects of chronic selective serotonin reuptake inhibitors on 8-OH-DPAT-induced facilitation of ejaculation in rats: comparison of fluvoxamine and paroxetine. Psychopharmacology (Berl). 2005; 179:509–515.6. Waldinger MD, Schweitzer DH, Olivier B. On-demand SSRI treatment of premature ejaculation: pharmacodynamic limitations for relevant ejaculation delay and consequent solutions. J Sex Med. 2005; 2:121–131.7. Atmaca M, Kuloglu M, Tezcan E, Ustundag B, Semercioz A. Serum leptin levels in patients with premature ejaculation before and after citalopram treatment. BJU Int. 2003; 91:252–254.8. Behre HM, Simoni M, Nieschlag E. Strong association between serum levels of leptin and testosterone in men. Clin Endocrinol (Oxf). 1997; 47:237–240.9. Atmaca M, Kuloglu M, Tezcan E, Semercioz A, Ustundag B, Ayar A. Serum leptin levels in patients with premature ejaculation. Arch Androl. 2002; 48:345–350.10. Nikoobakht MR, Tajik P, Karami AA, Moradi K, Mortazavi A, Kosari F. Premature ejaculation and serum leptin level: a diagnostic case-control study. J Sex Med. 2008; 5:2942–2946.11. Waldinger MD, Berendsen HH, Blok BF, Olivier B, Holstege G. Premature ejaculation and serotonergic antidepressants-induced delayed ejaculation: the involvement of the serotonergic system. Behav Brain Res. 1998; 92:111–118.12. McMahon CG, Althof SE, Waldinger MD, Porst H, Dean J, Sharlip ID, et al. An evidence-based definition of lifelong premature ejaculation: report of the International Society for Sexual Medicine (ISSM) ad hoc committee for the definition of premature ejaculation. J Sex Med. 2008; 5:1590–1606.13. Waldinger MD, Hengeveld MW, Zwinderman AH. Paroxetine treatment of premature ejaculation: a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 1994; 151:1377–1379.14. Ma Z, Gingerich RL, Santiago JV, Klein S, Smith CH, Landt M. Radioimmunoassay of leptin in human plasma. Clin Chem. 1996; 42(6 Pt 1):942–946.15. Crystal Chem Inc.Rat Leptin ELISA Kit [Internet]. Downers Grove, IL: Crystal Chem Inc.;c2010. cited 2014 May 26. Available from: http://www.crystalchem.com/rat-insulin-elisa-kit.html.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficacy and Safety of Paroxetine on the Treatment of Premature Ejaculation
- Serotonin Transporter Promoter Region (5-HTTLPR) Polymorphism Is Not Associated With Paroxetine-Induced Ejaculation Delay in Dutch Men With Lifelong Premature Ejaculation
- Recent Concepts of Premature Ejaculation
- The mathematical formula of the intravaginal ejaculation latency time (IELT) distribution of lifelong premature ejaculation differs from the IELT distribution formula of men in the general male population
- Selective Serotonin Reuptake Inhibitors for the Treatment of Premature Ejaculation