J Bacteriol Virol.
2015 Sep;45(3):256-261. 10.4167/jbv.2015.45.3.256.
Protein Expression of the Bombyx mori Decapentaplegic Gene using the Baculovirus Expression Vector System
- Affiliations
-
- 1Department of Biotechnology, Catholic University of Daegu, Daegu, Korea. microsw@cu.ac.kr
- KMID: 2068741
- DOI: http://doi.org/10.4167/jbv.2015.45.3.256
Abstract
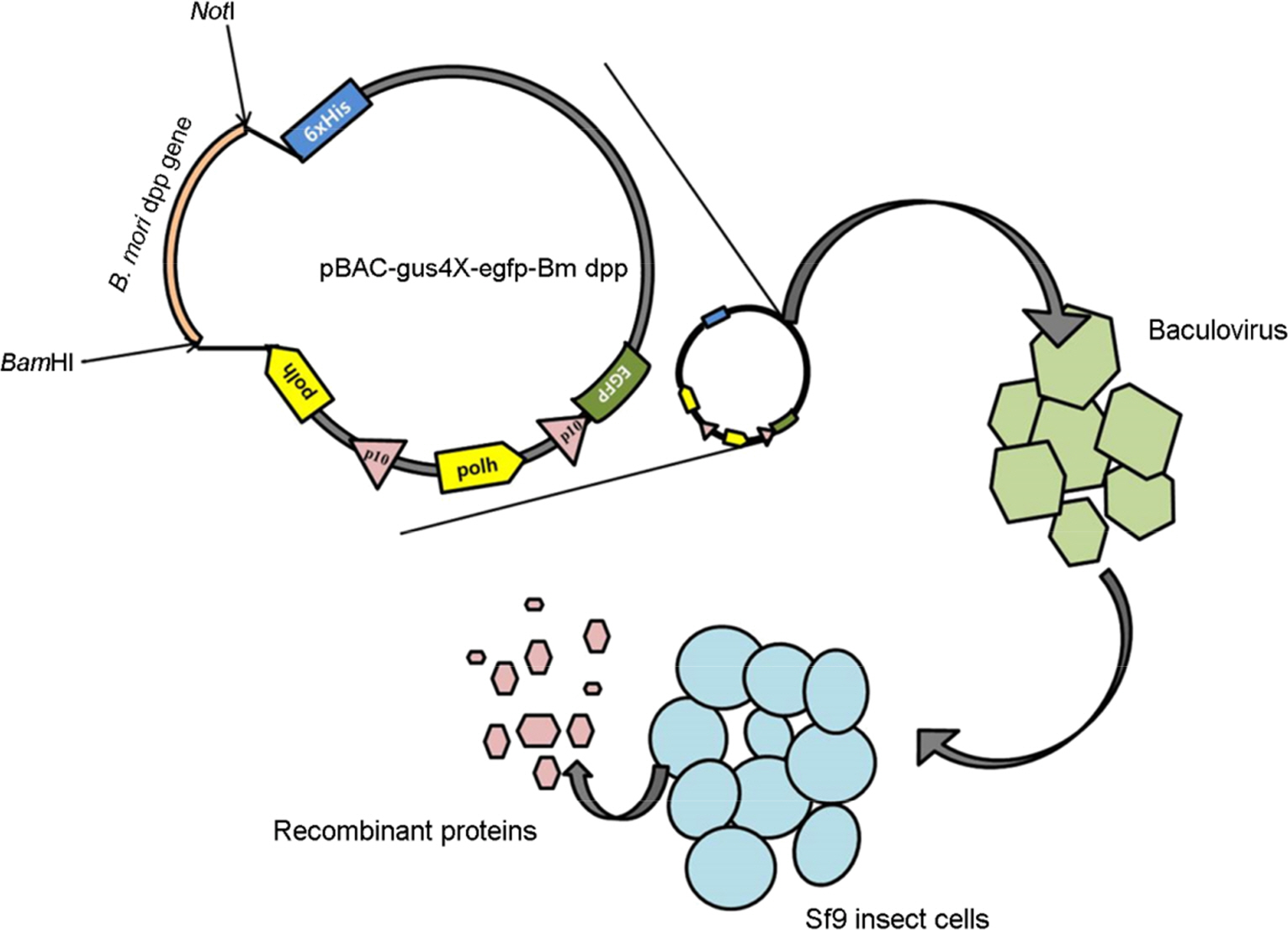
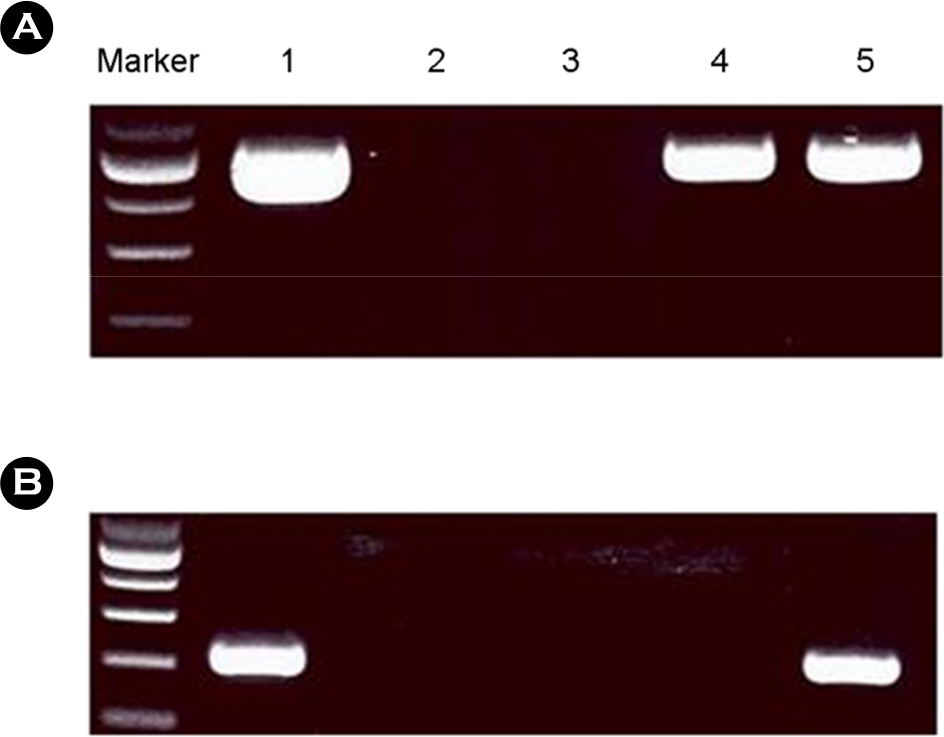
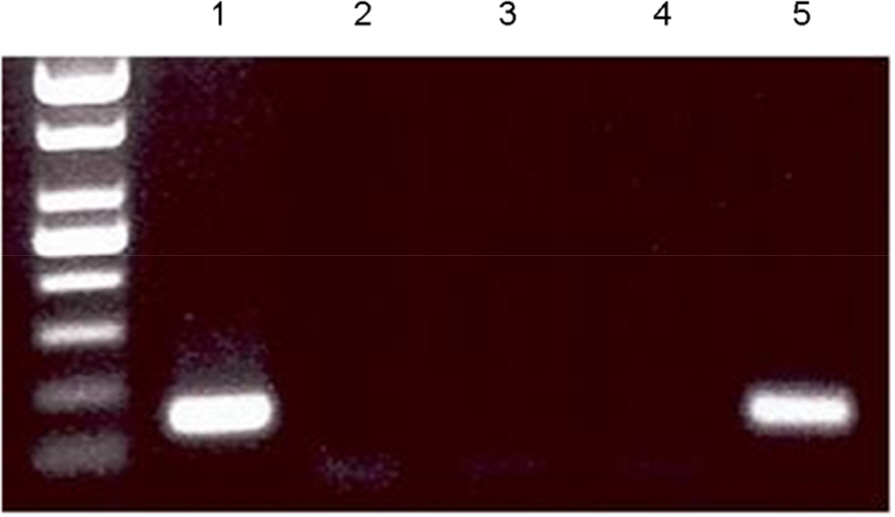
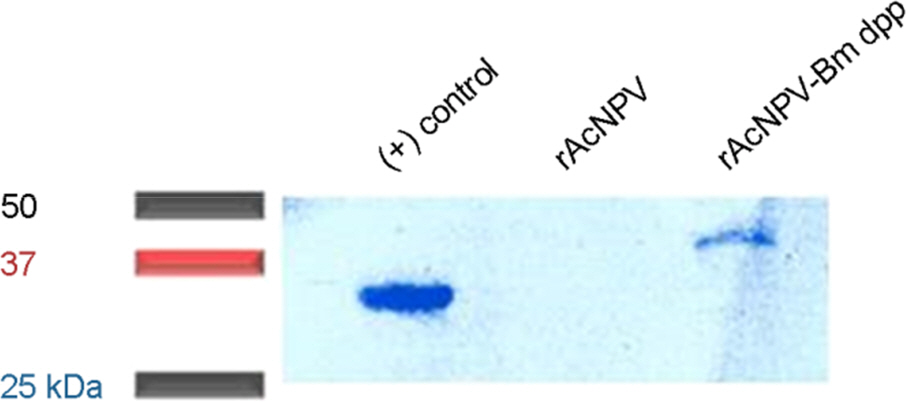
- The Bombyx mori decapentaplegic gene is one of the conserved genes in vertebrate and invertebrates. The TGF-beta superfamily contains conserved polypeptide growth factors that play important roles in different cellular processes such as proliferation, apoptosis, differentiation and cell-fate determination. The B. mori dpp gene shares genetic homology with hBMPs and Drosophila dpp. Until now, only few studies have been conducted to examine the functions of B. mori dpp; and hence, its function is not yet well understood. In this study, the baculovirus expression vector system (BEVS) was used for expression of the recombinant B. mori dpp protein and in which the recombinant baculovirus is recovered in the host Sf9 cells. The selected pure recombinant baculovirus containing B. mori dpp gene (rBV-egfp-Bm dpp) was used to increase the effective protein purification by using His-tag extraction strategy. After selection of recombinant baculovirus, recombinant B. mori dpp proteins were extracted from the re-infected cells with pure rBV-egfp-Bm dpp. Herein, we summarize the efficient expression and purification of B. mori dpp proteins from the insect cells using the BEVS. This recombinant protein could be suitable for functional test and various application studies.
MeSH Terms
Figure
Reference
-
1). Chen Y, Riese MJ, Killinger MA, Hoffmann FM. A genetic screen for modifiers of Drosophila decapentaplegic signaling identifies mutations in punt, Mothers against dpp and the BMP-7 homologue, 60A. Development. 1998; 125:1759–68.
Article2). Künnapuu J, Shimmi O. Evolutional imprints on the sequences of BMP2/4/DPP type proteins. Fly (Austin). 2010; 4:21–3.
Article3). Park SW, Goo TW, Choi GH, Kang SW, Kim SW, Kim SR. Functional analysis of Bombyx mori Decapentaplegic gene for bone differentiation in a mammalian cell. Int J Indust Entomol. 2013; 27:159–65.4). Park SW, Lee HK, Kim TG, Yoon SK, Paik SY. Hepatocyte-Specific Gene Expression by Baculovirus Pseudotyped with Vesicular Stomatitis Virus Envelope Glycoprotein. Biochem Biophys Res Commun. 2001; 289:444–50.
Article5). Jin JY, Park CJ, Park SW, Paik SY. Protein Expression of the Human Norovirus Capsid Gene using the Baculo-virus Expression System. J Bacteriol Virol. 2011; 41:183–7.
Article6). Russell D, Sambrook J. Molecular cloning: a laboratory manual. 3rd ed.Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press;2000.7). Sampath TK, Rashka KE, Doctor JS, Tucker RF, Hoffmann FM. Drosophila transforming growth factor beta superfamily proteins induce endochondral bone formation in mammals. Proc Natl Acad Sci U S A. 1993; 90:6004–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Green Fluorescent Protein in Both Spodoptera frugiperda Cells and Bombyx mori Larvae by Ac-Bm Hybrid Virus
- Mouse Dual Ig Domain Containing Cell Adhesion Molecule Protein Expression and Purification Using the Baculovirus Expression Vector System
- Expression of Phospholipase C-B3 using Recombinant Baculovirus Expression System
- Protein Expression of the Human Norovirus Capsid Gene using the Baculovirus Expression System
- Cloning and Expression of Hemagglutinin-Neuraminidase Gene of a Thermostable Isolate of Newcastle Disease Virus by Baculovirus Recombinants