J Bacteriol Virol.
2006 Sep;36(3):159-165. 10.4167/jbv.2006.36.3.159.
Antibiotic Resistance and Genotypic Characterization of Escherichia coli Isolated from Pig Farm Environment
- Affiliations
-
- 1Busan Metropolitan City Research Institute of Health & Environment, Busan, Korea.
- 2College of Veterinary Medicine, Kyungpook National University, Daegu, Korea. youngju@knu.ac.kr
- 3Daegu Metropolitan City Research Institute of Health & Environment, Daegu, Korea.
- 4National Veterinary Research and Quarantine Service, Anyang, 430-824, Korea.
- KMID: 2055023
- DOI: http://doi.org/10.4167/jbv.2006.36.3.159
Abstract
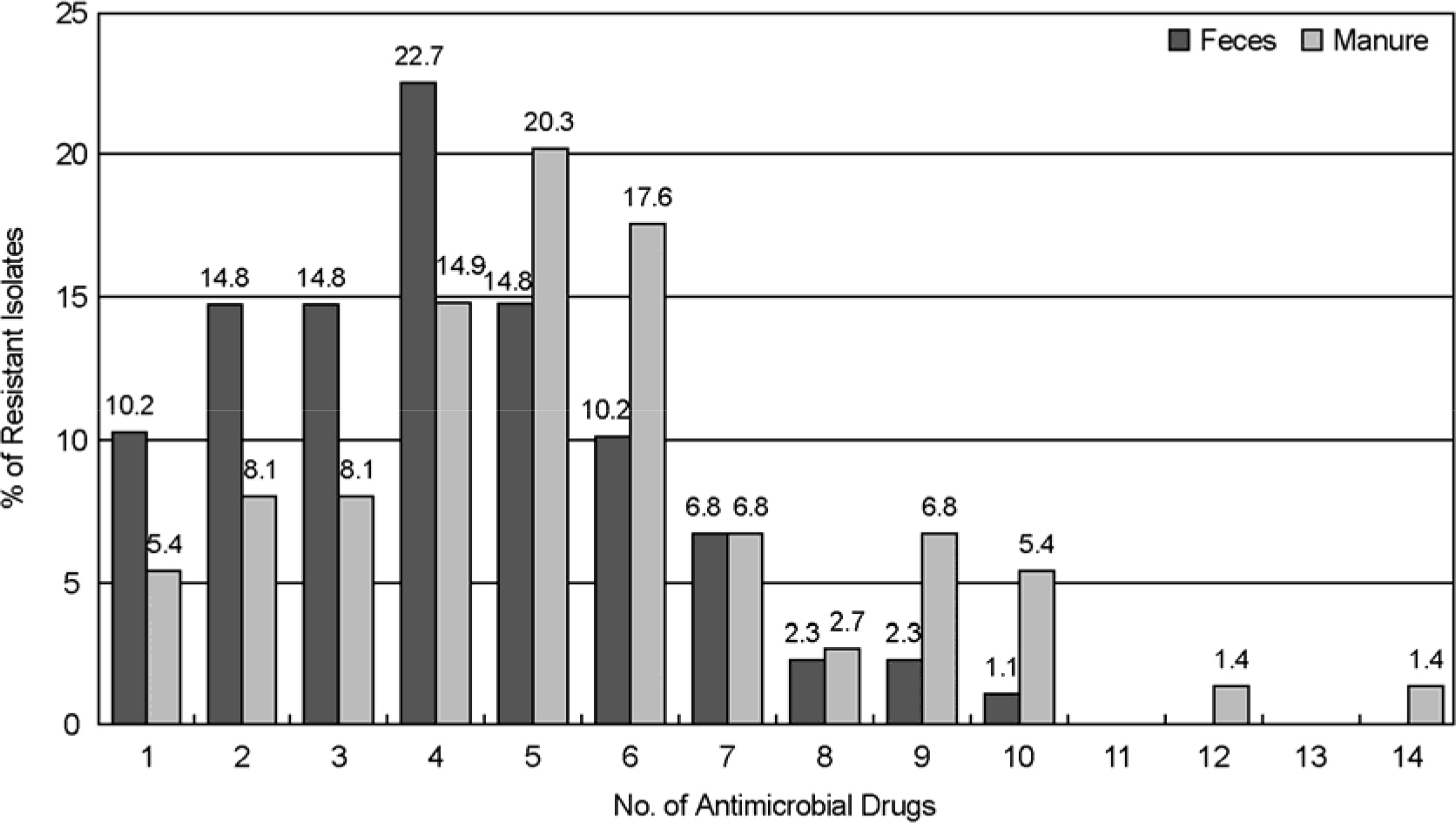
- The use of antibiotics, including therapeutically in human and veterinary medicine, or as prophylaxis of growth promotion in animal husbandry, ultimately exerts selective pressure favorable for the propagation of antibiotic resistant bacteria. In this study we have determined the resistance for antibiotics of E. coli from pig farm environment, and investigate genetic relatedness by random amplification of polymorphic DNA (RAPD). Six farms were randomly selected in Gyeongsanman-do and Busan provinces for collecting samples from feces, manure and underground water. A total of 88 isolates from feces, 74 isolates from manure and 1 isolate from underground water were analyzed by antibiotic resistance and RAPD. Antibiotic resistance testing was performed by disk diffusion method using 16 antibiotics. The highest percentage of antibiotic resistance of isolates from feces and manure was found to the following antibiotics; tetracycline (100% and 100%), sulfamethoxazole/trimethoprim (60.2% and 62.2%), streptomycin (50.0% and 68.9%), chloramphenicol (56.8% and 56.8%), ampicillin (50.0% and 81.1%) and cephalothin (50.0% and 51.4%). Of isolates from feces and manure, 22.7% and 20.3% showed multiple resistance to 4 and 5 antibiotics, respectively. The isolates from GE pig farm showed six RAPD patterns. A single pattern, RAPD-C, was predominat in feces isolates (50.0%) and manual isolates (46.7%), and the rest of feces isolates showed RADP-A, B and E pattern and manure isolates showed D and E pattern. One isolate from underground water showed F pattern. The appearance of multiresistant in E. coli isolates from pig farms environment is a problem of major concern of public health and RAPD may offer an useful tool of discrimination for the epidemiological investigation.
MeSH Terms
-
Ampicillin
Animal Husbandry
Anti-Bacterial Agents
Bacteria
Busan
Cephalothin
Chloramphenicol
Diffusion
Discrimination (Psychology)
DNA
Drug Resistance, Microbial*
Escherichia coli*
Escherichia*
Feces
Groundwater
Humans
Manure
Public Health
Streptomycin
Tetracycline
Veterinary Medicine
Ampicillin
Anti-Bacterial Agents
Cephalothin
Chloramphenicol
DNA
Manure
Streptomycin
Tetracycline
Figure
Reference
-
References
1). 국립수의과학검역원. 국립수의과학검역원 고시 제 2003 −14호: 축산물의 가공기준 및 성분규격. 150–181. 2002.2). 이연희. 임상과 축산에서 발견되는 항균제의 내성균주 의 현황. 한국수의공중보건학회 추계학술대회 특별연제. 43–54. 2003.3). 정석찬. 축산용항생제 관리시스템 구축. The annual report of KFDA. 8:2223–2224. 2004.4). 정윤희. 축산 환경 중의 항균제 내성균 모니터링. 식 품의약품안전청 용역최종보고서. 2003.5). 차인호. 설사환자로부터 분리한 대장균의 형별 분포 및 항균제 내성 유형. 생명과학회지. 6:262–272. 2000.6). 하준일, 홍기성, 송시욱, 정석찬, 민영식, 신형철, 이기 옥, 임경종, 박종명. 축산 및 수산분야의 항생물질 사용 실태 조사. 한국수의공중보건학회지. 27:205–217. 2003.7). 환경부. 환경부 고시 제 2002–91호: 먹는 물 수질오염 공정시험방법. 12–15. 2002.8). Avery SM, Liebana E, Reid CA, Woodward MJ, Buncic S. Combined use of two genetic fingerprinting methods, pulsed-field gel electrophoresis and ribotyping, for characterization of Escherichia coli O157:H7 isolates from food animals, retail meats, and cases of human disease. J Clin Microbiol. 40:2806–2812. 2002.9). Dermot JH, Helen HJ. Lessons from the Danish ban on feed-grade antibiotics. Briefing Paper. 03-BP41:1–9.10). Dunlop RH, McEwen SA, Meek AH, Clarke RC, Black WD, Friendship RM. Associations among antimicrobial drug treatments and antimicrobial resistance of fecal Escherichia coli of swine on 34 farrow-to-fininsh farms in Ontario, Canada. Prev Vet Med. 34:283–305. 1998.11). Hanzawa Y, Oka C, Ishiguro N, Sato G. Antibiotic-resistant coliforms in the waste of piggeries and dairy farms. Nippon Juigaku Zasshi. 46:363–372. 1984.
Article12). Harwood VJ, Brownell M, Perusek W, Whitlock JE. Vancomycin-resistant Enterococcus spp. isolated from wastewater and chicken feces in the United States. Appl Environ Microbiol. 67:4930–4933. 2001.13). Klare I, Badstubner D, Konstabel C, Bohme G, Claus H, Witte W. Decreased incidence of VanA-type vancomycin-resistant enterococci isolated from poultry meat and from fecal samples of humans in the community after discontinuation of avoparcin usage in animal husbandary. Microb Drug Resist. 5:45–52. 1999.14). Lanz R, Kuhnert P, Boerlin P. Antimicrobial resistance and resistance gene determinants in clinical Escherichia coli from different animal species in Switzerland. Vet Microbiol. 91:73–84. 2003.15). Loglois BE, Dawson KA, Leak I, Aeron DK. Antimicrobial resistance of fecal coliforms from pigs in a herd not exposed to antimicrobial agents for 126 months. Vet Microbiol. 18:147–153. 1988.16). Mathew AG, Saxton AM, Upchurch WG, Chattin SE. Multiple antibiotic resistance patterns of Escherichia coli isolates from swine farms. Appl Environ Microbiol. 65:2770–2772. 1999.17). Meyer KS, Urban C, Eagan JA, Berger BJ, Rahal JJ. Nosocomial outbreak of Klebsiella infection resistant late-generation cephalosporins. Ann Intern Med. 119:353–359. 1993.18). National Committee for Clinical Laboratory Standards. Performance strandards for antimicrobial disk susceptibility tests. 7th ed.M20A7;2000.19). Pacheco AB, Guth BE, Soares KC, Nishimura L, de Almeida DF, Ferreira LC. Random amplification of polymorphic DNA reveals serotype-specific clonal clusters among enterotoxigenic Escherichia coli strains isolated from human. J Clin Microbiol. 35:1521–1525. 1997.20). Raida SS, John BK, Yvette J, Roseann M. Patterns of antimicrobial resistance observed in Escherichia coli isolates obtained from domestic- and wild-animal fecal samples, human septage, and surface water. Appl Environ Microbiol. 71:1394–1404. 2005.21). Regua-Mangia AH, Guth BC, da Costa Andrade Jr, Irino K, Pacheco AB, Ferreira LC, Zahner V, Teixeira LM. Genotypic and phenotypic characterization of enterotoxigenic Escherichia coli (ETEC) strains isolated in Rio de Janeiro city, Brazil. FEMS Immunol Med Microbiol. 40:155–162. 2004.22). Saenz Y, Zarazaga M, Brinas L, Lantero M, Ruiz-Larrea F, Torres C. Antibiotic resistance in Escherichia coli isolates obtained from animals, food and humans in Spain. Int J Antimicrob Agents. 18:353–358. 2001.23). Sengelov G, Agerso Y, Halling-Sorensen B, Baloda SB, Andersen JS, Jensen LB. Bacterial antibiotic resistance levels in Danish farmland as a result of treatment with pig manure slurry. Environ Int. 28:587–595. 2003.24). van den Bogaard AE, Bruinsma N, Stobberingh EE. The effect of banning avoparcin on VRE carriage in The Netherlands. J Antimicrob Chemother. 46:146–148. 2000.
Article25). Whittam TS, Wachsmuth IK, Wilson RA. Genetic evidence of clonal descent of Escherichia coli O157:H7 associated with hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 157:1124–1233. 1988.26). WHO. Use of antimicrobials outside human medicine and resultant antimicribial resistance in humans. Fact Sheet No. 268:. 2002.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of antibiotic resistance profiles for Escherichia coli isolated from wild boar and domestic pig fecal samples
- Whole genome sequencing analysis Microbiology on antibiotic-resistant Escherichia coli isolated from pig farms in Banten Province, Indonesia
- Comparison of Antimicrobial Resistance in
Escherichia coli Strains Isolated From Healthy Poultry and Swine Farm Workers Using Antibiotics in Korea - Characterization of Antimicrobial Susceptibility, Extended-Spectrum β-Lactamase Genes and Phylogenetic Groups of Enteropathogenic Escherichia coli Isolated from Patients with Diarrhea
- Antimicrobial Resistance in Escherichia coli Isolated from Healthy Volunteers of the Community