Lab Med Online.
2013 Jul;3(3):160-168. 10.3343/lmo.2013.3.3.160.
Performance Evaluation of 3 Kinds of HBsAg Qualitative Assays and 2 Kinds of Quantitative Assays
- Affiliations
-
- 1Department of Laboratory Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea. srkimuuh@hitel.net
- 2Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea.
- 3Department of General Surgery, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea.
- 4Department of Laboratory Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2053551
- DOI: http://doi.org/10.3343/lmo.2013.3.3.160
Abstract
- BACKGROUND
Currently used techniques for quantitation of HBsAg often yield discordant results; therefore, development of quantitation techniques that can detect HBsAg with high accuracy has become very important. Recent advances have led to the development of several HBsAg detection systems. Here, we evaluated the performance of 3 newly developed detection systems, which can detect HBsAg both qualitatively and quantitavely, and determined the concordance among their results.
METHODS
Four hundred and thirty two samples assigned to 4 groups-patient group, dilution group, weakly reactive group, and linearity group- were subjected to qualitative and quantitative detection of HBsAg by using the 3 systems developed by 3 major manufacturers; Abbott Architect, Roche E170 and Siemens Centaur XP.
RESULTS
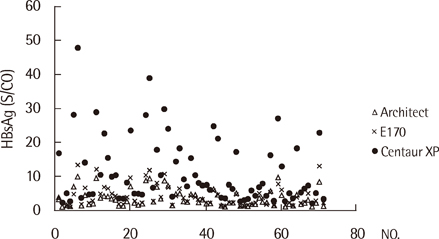
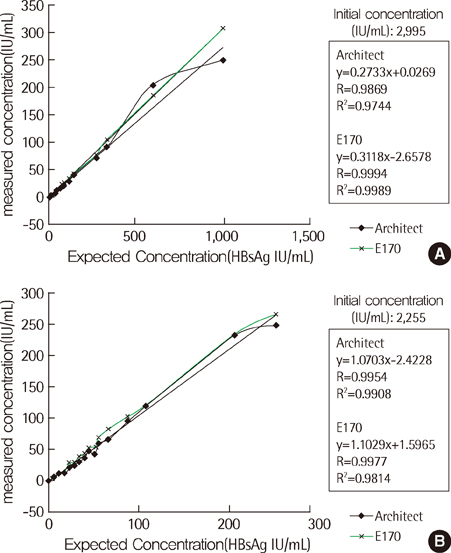
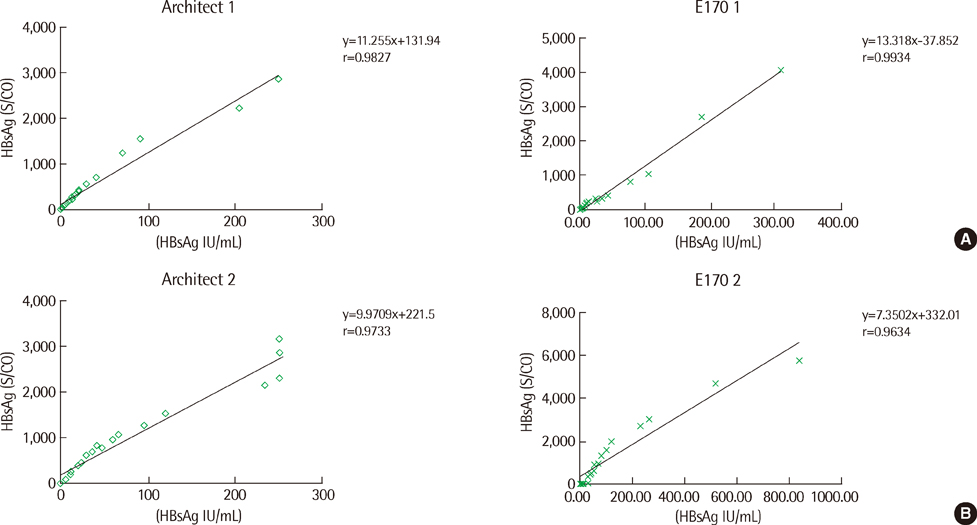
The results for the qualitative analyses were closely concordant among the three systems (98.3%) for all 432 samples. In 123 samples that were determined as HBsAg-negative, E170 (76%) distributed frequently at the upper half level (0.5-1.0) of negative reference range, compared with Architect (11%) and Centaur XP (22%). In particular, in 65 samples that were diluted from the strongly positive samples to obtain weakly positive samples, the average index values obtained using Architect (3.6 S/CO), E170 (4.2 COI) and Centaur XP (11.4 index value) differed significantly (P<0.0001). In the antiviral treatment group and the post-liver transplantation group, no inconsistency was observed among the results of the qualitative and quantitative assays. In the 18-fold serially diluted samples, no linearity was observed.
CONCLUSIONS
Because of the possibility of false-positive detection in the HBsAg-negative samples, regular management of equipment and appropriate selection of reagents are very important. In weakly positive samples, quantitative assay has not to be replaced for qualitative assay. Therefore, the qualitative assays should be used for screening the samples, whereas the quantitative assays should be used for monitoring the Hepatitis B virus (HBV) load in the samples determined as HBsAg-positive. The qualitative index value should not be interpreted as a quantitative measure of HBV load.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee WM. Hepatitis B virus infection. N Engl J Med. 1997; 337:1733–1745.
Article2. Lee HS. Current status of viral hepatitis and serological diagnosis. Korean J Clin Pathol. 1995; 15(S2):S197–S212.3. Lee DH, Kim JH, Nam JJ, Kim HR, Shin HR. Epidemiological findings of hepatitis B infection based on 1998 National Health and Nutrition Survey in Korea. J Korean Med Sci. 2002; 17:457–462.
Article4. Su TH, Hsu CS, Chen CL, Liu CH, Huang YW, Tseng TC, et al. Serum hepatitis B surface antigen concentration correlates with HBV DNA level in patients with chronic hepatitis B. Antivir Ther. 2010; 15:1133–1139.
Article5. Wursthorn K, Lutgehetmann M, Dandri M, Volz T, Buggisch P, Zollner B, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. 2006; 44:675–684.
Article6. Chen CH, Lee CM, Wang JH, Tung HD, Hung CH, Lu SN. Correlation of quantitative assay of hepatitis B surface antigen and HBV DNA levels in asymptomatic hepatitis B virus carriers. Eur J Gastroenterol Hepatol. 2004; 16:1213–1218.
Article7. Jilg W, Sieger E, Zachoval R, Schatzl H. Individuals with antibodies against hepatitis B core antigen as the only serological marker for hepatitis B infection: high percentage of carriers of hepatitis B and C virus. Journal of Hepatology. 1995; 23:14–20.
Article8. Coleman PF, Chen YC, Mushahwar IK. Immunoassay detection of hepatitis B surface antigen mutants. J Med Virol. 1999; 59:19–24.
Article9. Weber B, Dengler T, Berger A, Doerr HW, Rabenau H. Evaluation of two new automated assays for hepatitis B virus surface antigen (HBsAg) detection: IMMULITE HBsAg and IMMULITE 2000 HBsAg. J Clin Microbiol. 2003; 41:135–143.
Article10. Moerman B, Moons V, Sommer H, Schmitt Y, Stetter M. Evaluation of sensitivity for wild type and mutant forms of hepatitis B surface antigen by four commercial HBsAg assays. Clin Lab. 2004; 50:159–162.11. Weber B. Genetic variability of the S gene of hepatitis B virus: clinical and diagnostic impact. J Clin Virol. 2005; 32:102–112.
Article12. Song SM, Oh WI, Kim DW. Evaluation of Serologic Marker Tests for Hepatitis B Viral Infection Using the Automated Immunoassay System ARCHITECT i2000. Korean J Clin Pathol. 2002; 22:42–46.13. Yoo SJ, Oh HJ, Shin BM. Comparison of 3 Automated Immunoassay for Hepatitis B surface Antigen. Korean J Lab Med. 2006; 26:282–286.
Article14. Cha YJ, Kwon SY, Kim TY, Kim JR, Kim HS, Park MH, et al. Annual report on external quality assessment in immunoserology in Korea(2005). J Lab Med Qual Assur. 2006; 28:41–61.15. Cha YJ, Kwon SY, Kim TY, Kim JR, Kim HS, Park MH, et al. Annual report on external quality assessment in immunoserology in Korea(2006). J Lab Med Qual Assur. 2007; 29:45–64.16. Cha YJ, Kwon SY, Kim TY, Kim JR, Kim HS, Park MH, et al. Annual report on external quality assessment in immunoserology in Korea(2007). J Lab Med Qual Assur. 2008; 30:49–74.17. Cha YJ, Kwon SY, Kim TY, Kim JR, Kim HS, Park MH, et al. Annual report on external quality assessment in immunoserology in Korea(2008). J Lab Med Qual Assur. 2009; 31:49–72.18. Chen D, Kaplan L, Liu Q. Evaluation of two chemiluminescent immunoassays of ADVIA Centaur for hepatitis B serology markers. Clin Chim Acta. 2005; 355:41–45.
Article19. Taylor P, Pickard G, Gammie A, Atkins M. Comparison of the ADVIA Centaur and Abbott AxSYM immunoassay systems for a routine diagnostic virology laboratory. J Clin Virol. 2004; 30:Suppl 1. S11–S15.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of 3 Automated Immunoassays for Hepatitis B Surface Antigen
- Comparison of Three Assay Systems for Qualitative and Quantitative Results of Hepatitis B Surface Antibody
- Increasing the Efficiency of Laboratory Performance by Using the Onboard Dilution Algorithm of the Elecsys Hepatitis B Surface Antigen II Quantitative Assay
- Evaluation of Abbott Fourth Generation HIV Antigen and Antibody Assays
- Evaluation of HBs Ag, HCV and HIV Ag-Ab Assays using Bio-Rad Elite Microplate Analyzer