Electrolyte Blood Press.
2007 Dec;5(2):75-88. 10.5049/EBP.2007.5.2.75.
Immunolocalization of Protein Kinase C Isoenzymes alpha, betaI, betaII and gamma in Adult and Developing Rat Kidney
- Affiliations
-
- 1Department of Anatomy and MRC for Cell Death Disease Research Center, The Catholic University of Korea College of Medicine, Seoul, Korea. jinkim@catholic.ac.kr
- 2Department of Internal Medicine, Sungkyunkwan University, Kangbuk Samsung Hospital, Seoul, Korea.
- KMID: 2052289
- DOI: http://doi.org/10.5049/EBP.2007.5.2.75
Abstract
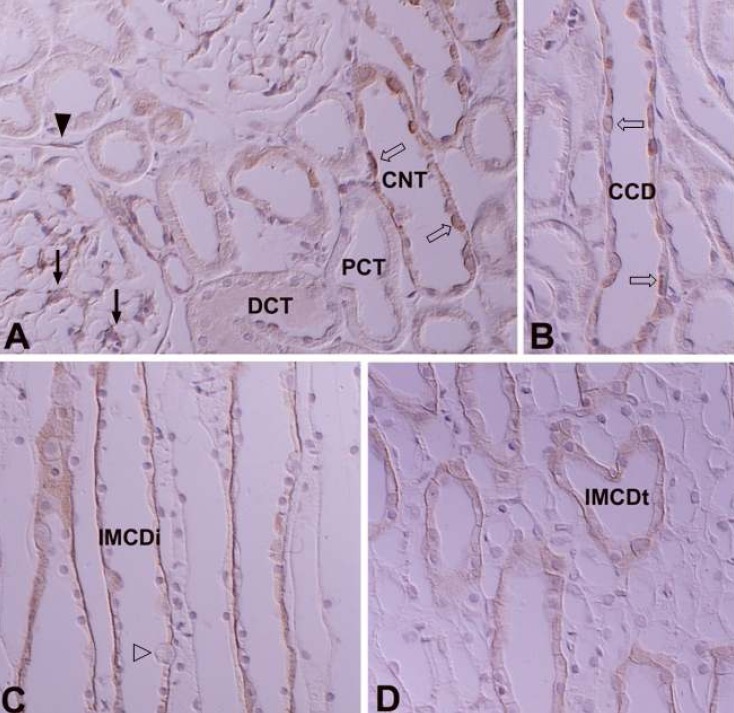
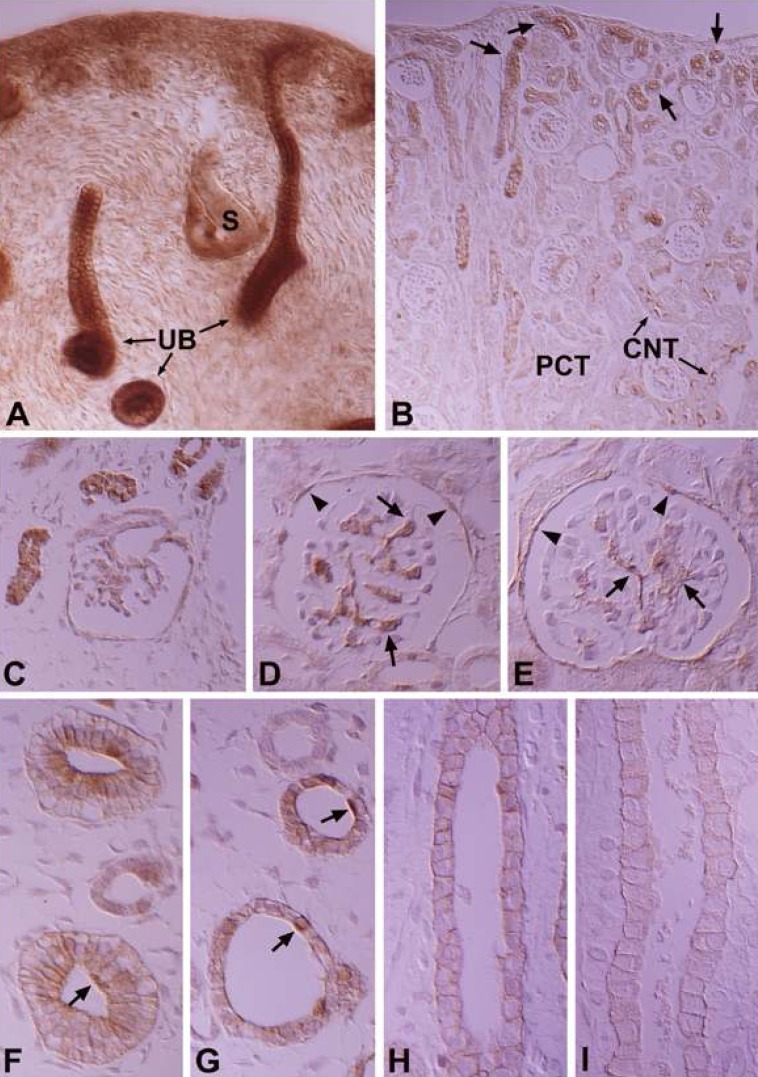
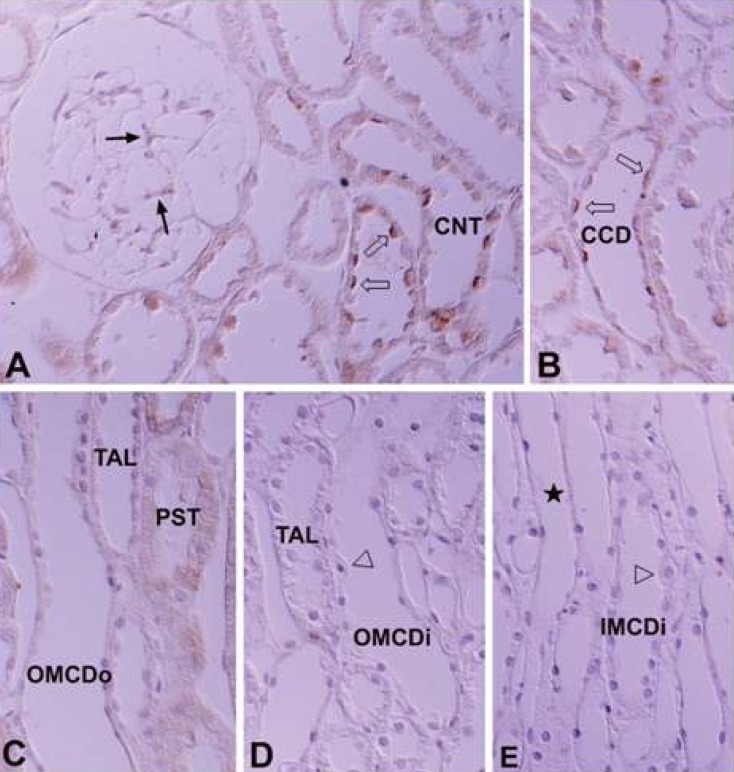
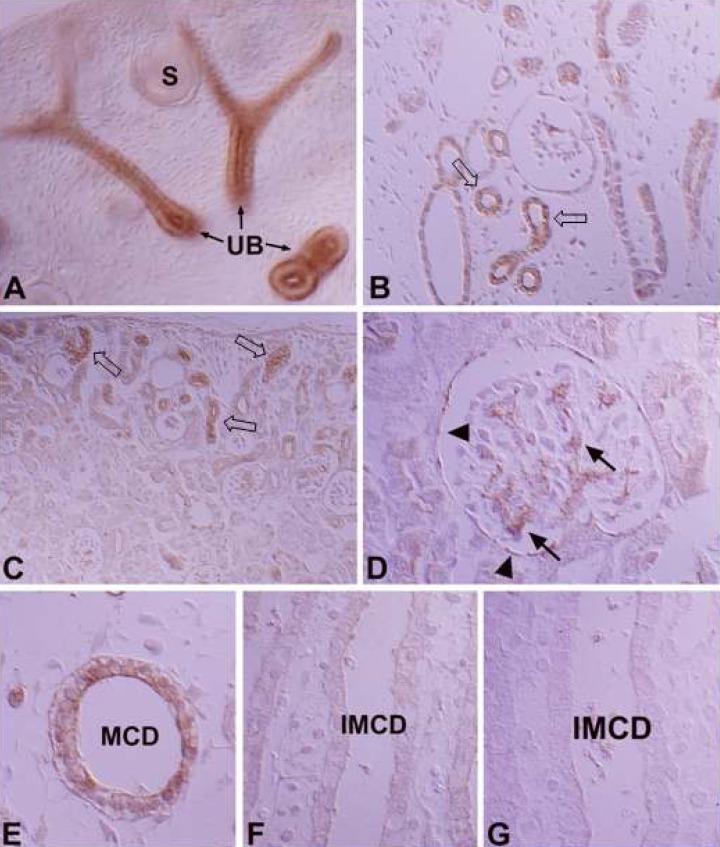
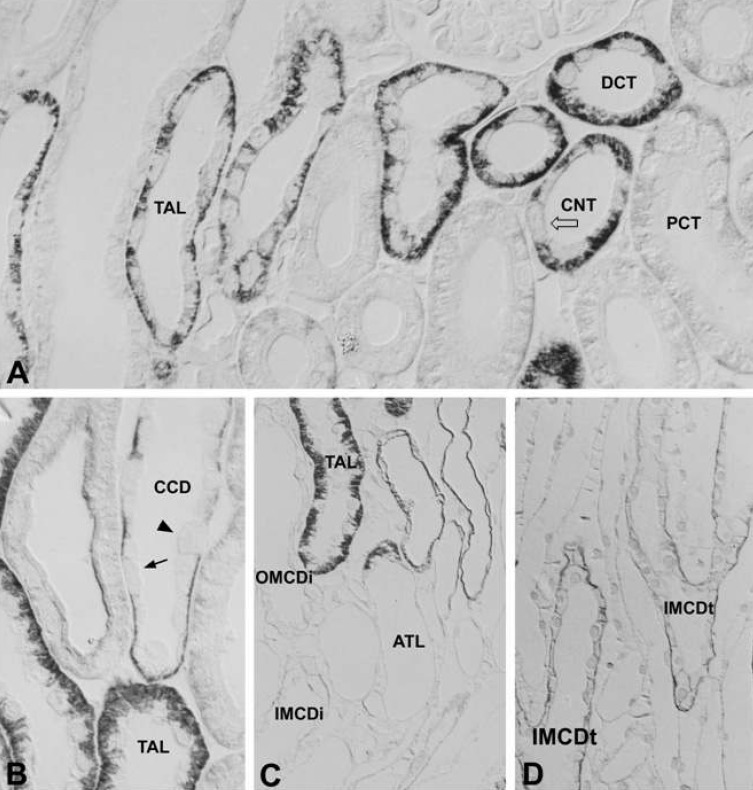
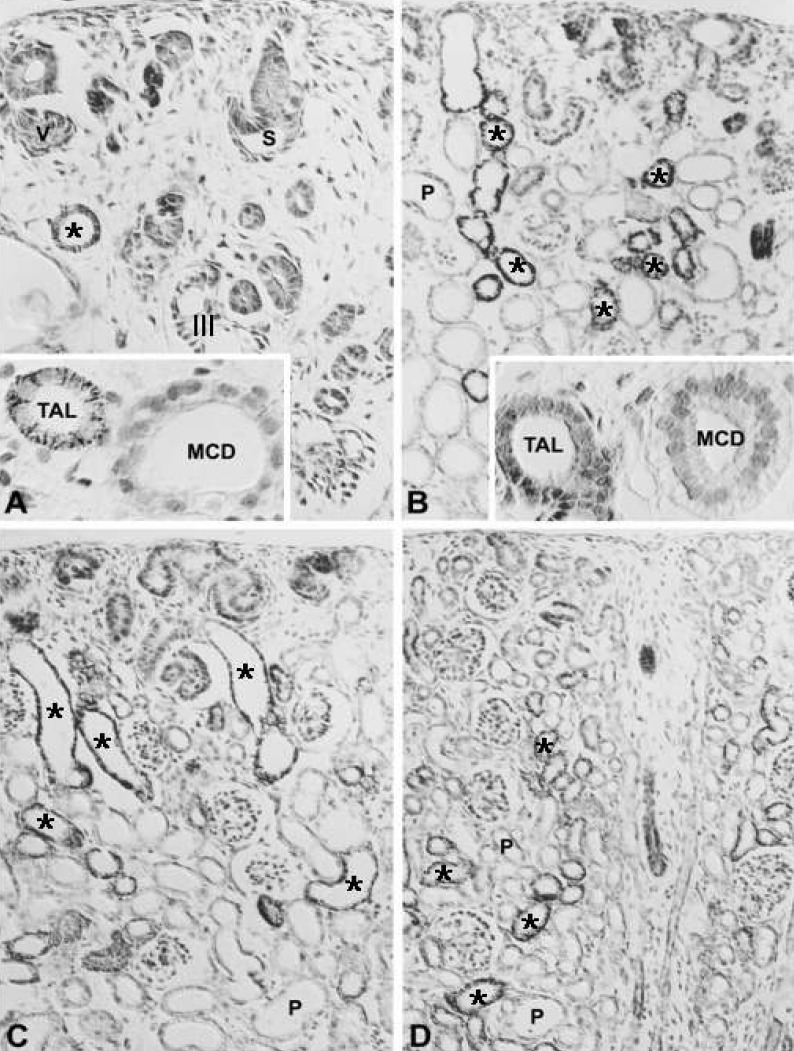
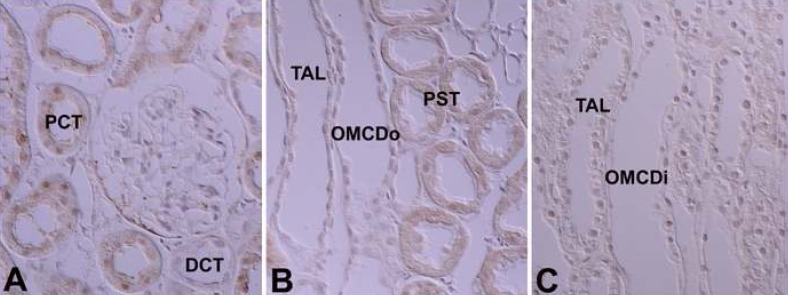
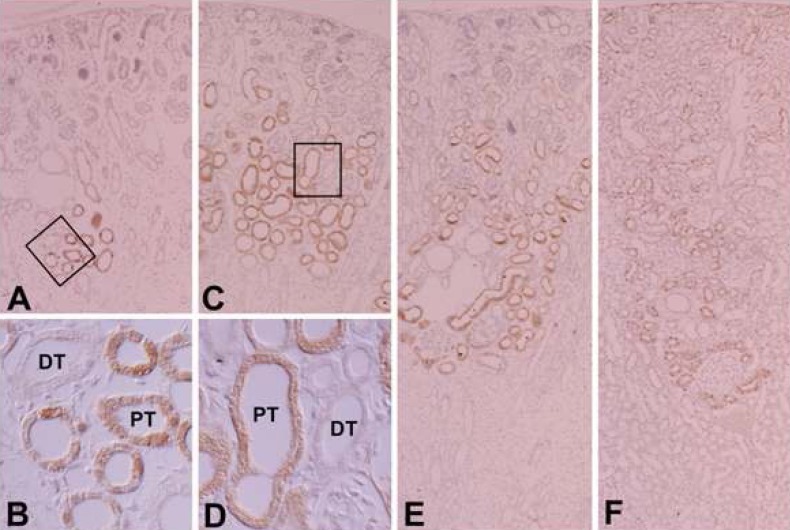
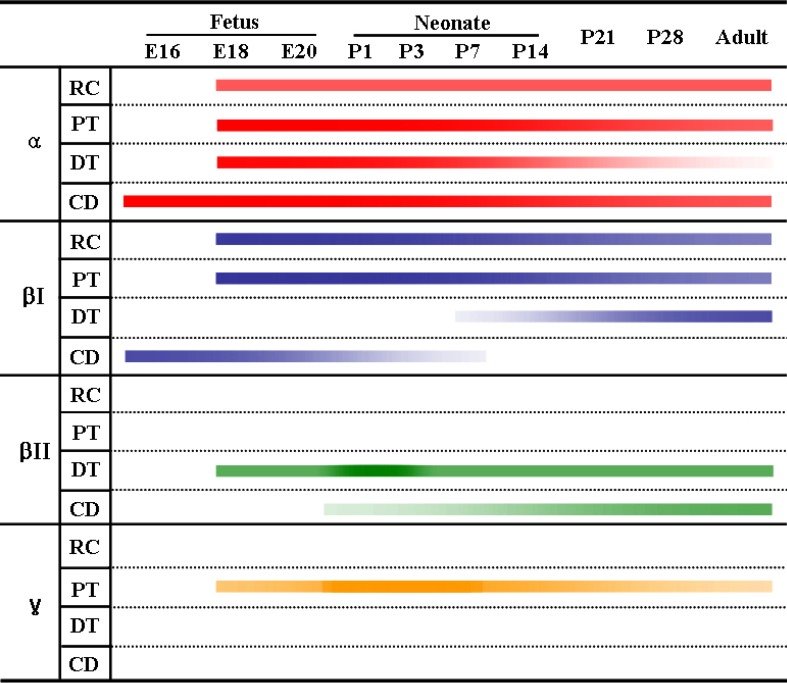
- Protein kinase C (PKC) plays an important role not only in signal transduction mechanisms in various biological processes, but also in the regulation of growth and differentiation during development. We studied the classical PKC alpha, betaI, betaII and gamma, with regard to their expression in adult and developing rat kidney. PKCalpha appeared in the ureteric bud at embryonic day (E) 16, and the proximal and distal anlage at E18. After birth, the immunoreactivity of PKCalpha gradually decreased. In adult, PKCalpha was expressed intensely in the connecting tubule (CNT), the collecting ducts (CD) and the renal corpuscle, and weakly in the proximal and distal tubules. PKCbetaI appeared in the ureteric bud at E16, and the proximal anlage at E18. After birth, the immunoreactivity of PKCbetaI gradually disappeared from the CD and proximal tubule. In adult, PKCbetaI was expressed in the intercalated cells of the CNT and cortical CD, the proximal straight tubule, and the renal corpuscle. PKCbII appeared in distal anlage at E18, and increased markedly after birth. In the CD, PKCbetaII immunoreactivity appeared after birth. In adult, PKCbetaII was expressed in the distal tubule, the CNT and the CD. The immunoreactivity for PKCgamma appeared only in the proximal anlage at E18, and increased temporally around the time of birth. However, no immunoreactivity for PKCgamma was observed in adult rat kidney. These results indicate that classical PKC isoforms appear to play a role in the regulation of various renal functions and differentiation within specific functional units of the uriniferous tubule in rat kidney.
MeSH Terms
Figure
Reference
-
1. Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986; 233:305–312. PMID: 3014651.
Article2. Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992; 258:607–614. PMID: 1411571.
Article3. Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995; 9:484–496. PMID: 7737456.
Article4. Bertorello AM, Aperia A, Walaas SI, Nairn AC, Greengard P. Phosphorylation of the catalytic subunit of Na+, K(+)-ATPase inhibits the activity of the enzyme. Proc Natl Acad Sci U S A. 1991; 88:11359–11362. PMID: 1662394.5. Nowicki S, Kruse MS, Brismar H, Aperia A. Dopamine-induced translocation of protein kinase C isoforms visualized in renal epithelial cells. Am J Physiol Cell Physiol. 2000; 279:C1812–C1818. PMID: 11078696.
Article6. Middleton JP, Khan WA, Collinsworth G, Hannun YA, Medford RM. Heterogeneity of protein kinase C-mediated rapid regulation of Na/K-ATPase in kidney epithelial cells. J Biol Chem. 1993; 268:15958–15964. PMID: 8393456.
Article7. Horie S, Moe O, Miller RT, Alpern RJ. Long-term activation of protein kinase c causes chronic Na/H antiporter stimulation in cultured proximal tubule cells. J Clin Invest. 1992; 89:365–372. PMID: 1310692.
Article8. Ruiz OS, Arruda JA. Regulation of the renal NaHCO3 cotransporter by cAMP and Ca-dependent protein kinases. Am J Physiol. 1992; 262:F560–F565. PMID: 1314505.9. Kosaka Y, Ogita K, Ase K, Nomura H, Kikkawa U, Nishizuka Y. The heterogeneity of protein kinase C in various rat tissues. Biochem Biophys Res Commun. 1988; 151:973–981. PMID: 3355565.
Article10. Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988; 263:6927–6932. PMID: 2834397.
Article11. Wetsel WC, Khan WA, Merchenthaler I, Rivera H, Halpern AE, Phung HM, Negro-Vilar A, Hannun YA. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J Cell Biol. 1992; 117:121–133. PMID: 1556149.
Article12. La Porta CA, Comolli R. Biochemical and immunological characterization of calcium-dependent and -independent PKC isoenzymes in renal ischemia. Biochem Biophys Res Commun. 1993; 191:1124–1130. PMID: 8466490.13. Aristimuño PC, Good DW. PKC isoforms in rat medullary thick ascending limb: selective activation of the delta-isoform by PGE2. Am J Physiol. 1997; 272:F624–F631. PMID: 9176373.14. Ostlund E, Mendez CF, Jacobsson G, Fryckstedt J, Meister B, Aperia A. Expression of protein kinase C isoforms in renal tissue. Kidney Int. 1995; 47:766–773. PMID: 7752575.15. Serlachius E, Svennilson J, Schalling M, Aperia A. Protein kinase C in the developing kidney: isoform expression and effects of ceramide and PKC inhibitors. Kidney Int. 1997; 52:901–910. PMID: 9328928.
Article16. Redling S, Pfaff IL, Leitges M, Vallon V. Immunolocalization of protein kinase C isoenzymes alpha, beta I, beta II, delta, and epsilon in mouse kidney. Am J Physiol Renal Physiol. 2004; 287:F289–F298. PMID: 15039141.17. Hashimoto T, Ase K, Sawamura S, Kikkawa U, Saito N, Tanaka C, Nishizuka Y. Postnatal development of a brain-specific subspecies of protein kinase C in rat. J Neurosci. 1988; 8:1678–1683. PMID: 3367216.
Article18. Hirata M, Saito N, Kono M, Tanaka C. Differential expression of the beta I- and beta II-PKC subspecies in the postnatal developing rat brain; an immunocytochemical study. Brain Res Dev Brain Res. 1991; 62:229–238.19. Pucéat M, Hilal-Dandan R, Strulovici B, Brunton LL, Brown JH. Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J Biol Chem. 1994; 269:16938–16944. PMID: 8207017.
Article20. Rybin VO, Steinberg SF. Protein kinase C isoform expression and regulation in the developing rat heart. Circ Res. 1994; 74:299–309. PMID: 8293569.
Article21. Housey GM, Johnson MD, Hsiao WL, O'Brian CA, Murphy JP, Kirschmeier P, Weinstein IB. Overproduction of protein kinase C causes disordered growth control in rat fibroblasts. Cell. 1988; 52:343–354. PMID: 3345563.22. Dong L, Stevens JL, Fabbro D, Jaken S. Regulation of protein kinase C isozymes in kidney regeneration. Cancer Res. 1993; 53:4542–4549. PMID: 8402625.23. Mischak H, Goodnight JA, Kolch W, Martiny-Baron G, Schaechtle C, Kazanietz MG, Blumberg PM, Pierce JH, Mushinski JF. Overexpression of protein kinase C-delta and -epsilon in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993; 268:6090–6096. PMID: 8454583.
Article24. Berra E, Diaz-Meco MT, Dominguez I, Municio MM, Sanz L, Lozano J, Chapkin RS, Moscat J. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell. 1993; 74:555–563. PMID: 7688666.25. Szallasi Z, Kosa K, Smith CB, Dlugosz AA, Williams EK, Yuspa SH, Blumberg PM. Differential regulation by anti-tumor-promoting 12-deoxyphorbol-13-phenylacetate reveals distinct roles of the classical and novel protein kinase C isozymes in biological responses of primary mouse keratinocytes. Mol Pharmacol. 1995; 47:258–265. PMID: 7870033.26. Pfaff IL, Wagner HJ, Vallon V. Immunolocalization of protein kinase C isoenzymes alpha, beta1 and betaII in rat kidney. J Am Soc Nephrol. 1999; 10:1861–1873. PMID: 10477137.27. Saxena R, Saksa BA, Hawkins KS, Ganz MB. Protein kinase C beta I and beta II are differentially expressed in the developing glomerulus. FASEB J. 1994; 8:646–653. PMID: 8005392.28. Dong LQ, Stevens JL, Jaken S. Biochemical and immunological characterization of renal protein kinase C. Am J Physiol. 1991; 261:F679–F687. PMID: 1928379.
Article29. Fukuzaki A, Kaneto H, Ikeda S, Orikasa S. Characterization of protein kinase C expression in human kidney. Tohoku J Exp Med. 1996; 178:263–269. PMID: 8727708.
Article30. Madsen KM, Tisher CC. Structural-functional relationships along the distal nephron. Am J Physiol. 1986; 250:F1–F15.
Article31. Kim WY, Jung JH, Park EY, Yang CW, Kim H, Nielsen S, Madsen KM, Kim J. Expression of protein kinase C isoenzymes alpha, betaI, and delta in subtypes of intercalated cells of mouse kidney. Am J Physiol Renal Physiol. 2006; 291:F1052–F1060. PMID: 16735462.32. Madsen KM, Verlander JW, Kim J, Tisher CC. Morphological adaptation of the collecting duct to acidbase disturbances. Kidney Int. 1991; 40(Suppl 33):S57–S63.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Protein Kinase C Isoform mRNAs in the Developing Rat Heart
- The expression of Protein Kinase C Isozymes in the Rat Brain
- Ischemic Preconditioning and the Role of Protein Kinase C in Cultured Retinal Ganglion Cell Line
- Effects of Epigallocatechin-3-Gallate on the Expression of TGF-beta1, PKC alpha/betaII, and NF-kappaB in High-Glucose-Stimulated Glomerular Epithelial Cells
- Modulation of Muscarinic K+ Channel by Protein Kinase C in Ischemic Rat Atrial Myocytes