Anat Cell Biol.
2013 Dec;46(4):272-284. 10.5115/acb.2013.46.4.272.
Expression of carbonic anhydrase IX in human fetal joints, ligaments and tendons: a potential marker of mechanical stress in fetal development?
- Affiliations
-
- 1Department of Anatomy, Chonbuk National University Medical School, Jeonju, Korea.
- 2Department of Anatomy, Institute of Bioscience and Medical Technology, University of Tampere, Tampere, Finland.
- 3Maxillofacial Anatomy, Department of Maxillofacial Biology, Tokyo Medical and Dental University Graduate School, Tokyo, Japan.
- 4Department of Anatomy, Sapporo Medical University School of Medicine, Sapporo, Japan.
- 5Division of Internal Medicine, Iwamizawa Kojin-kai Hospital, Iwamizawa, Japan.
- 6Department of Surgery and Research Institute of Clinical Medicine, Chonbuk National University Hospital, Chonbuk National University Medical School, Jeonju, Korea. chobh@jbnu.ac.kr
- KMID: 2046768
- DOI: http://doi.org/10.5115/acb.2013.46.4.272
Abstract
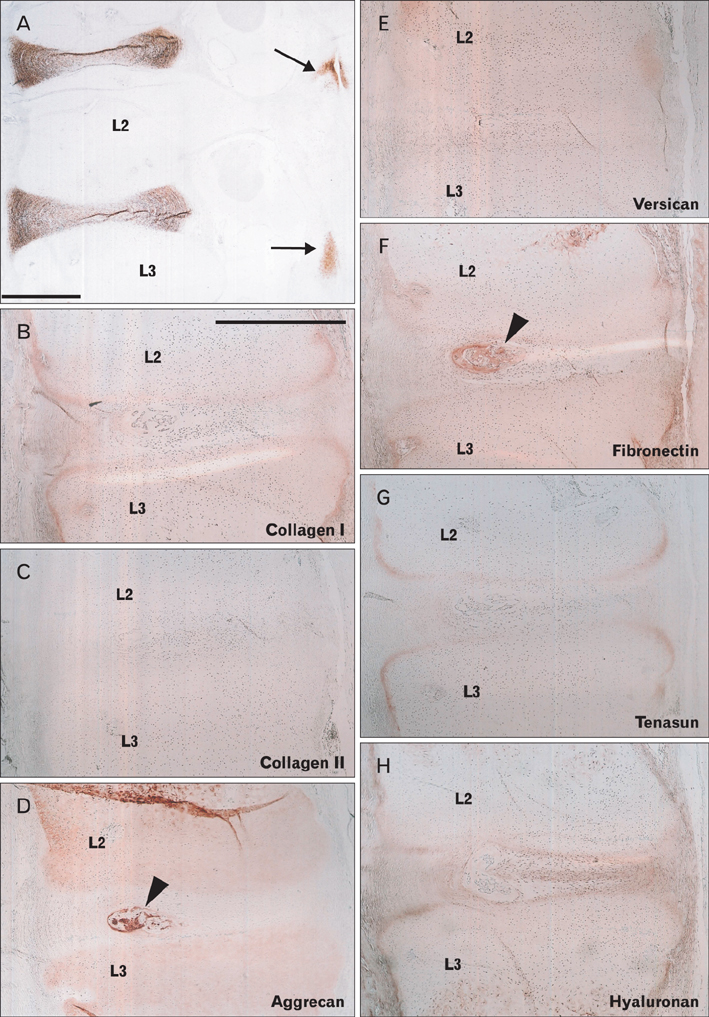
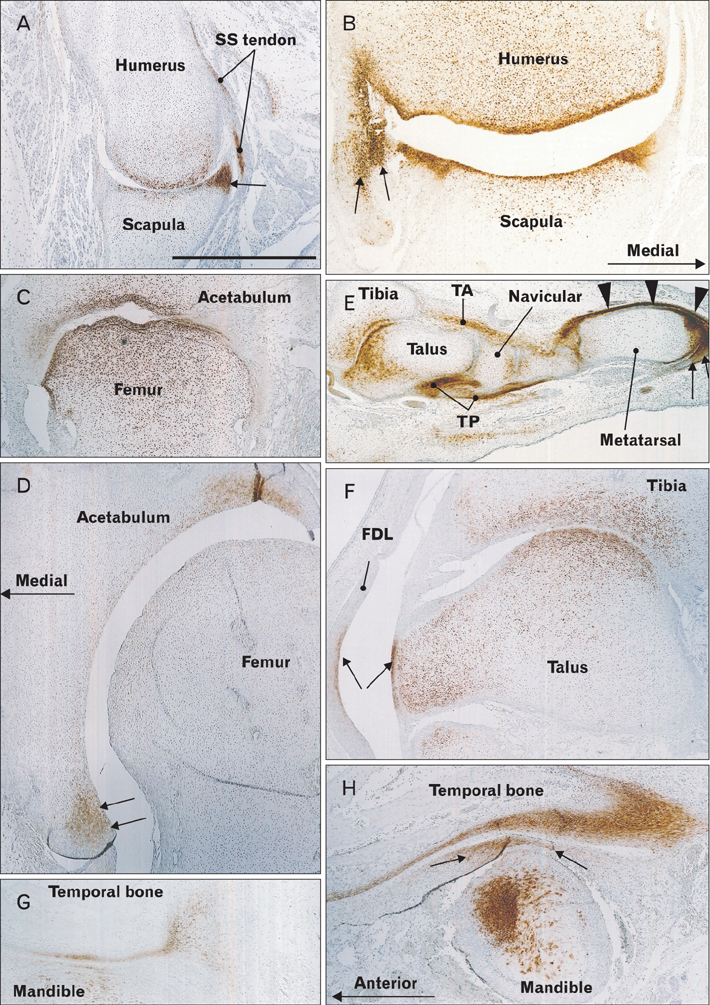
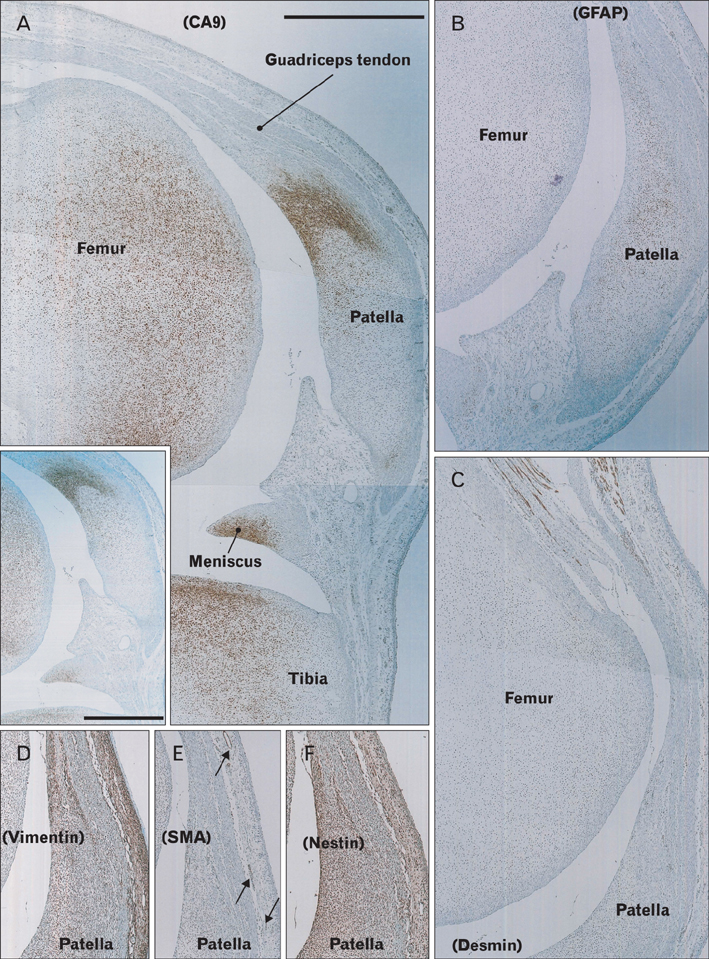
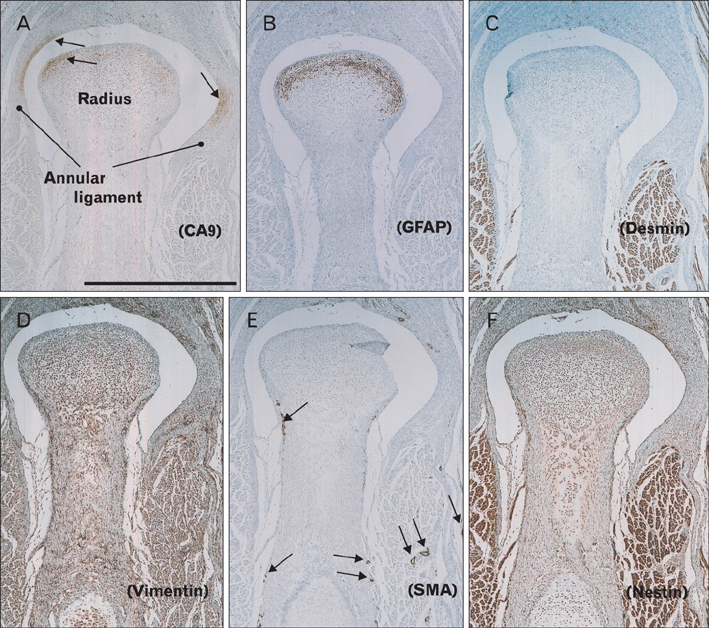
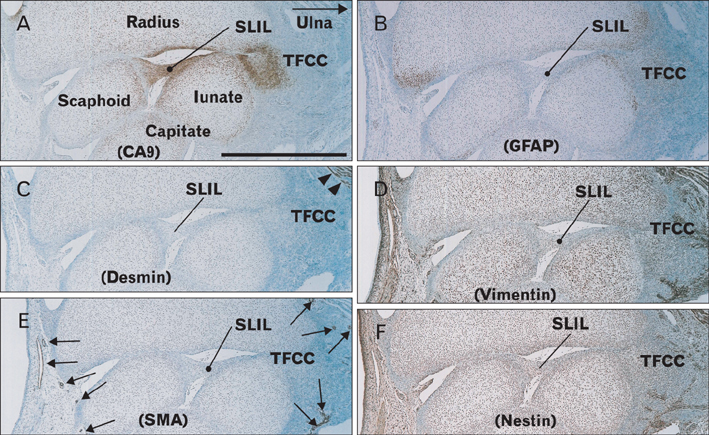
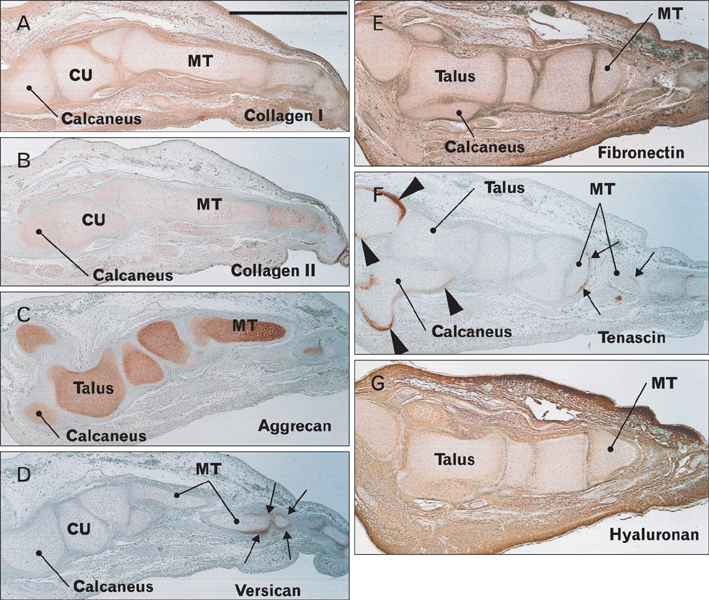
- Carbonic anhydrase type IX (CA9) is known to express in the fetal joint cartilage to maintain pH against hypoxia. Using paraffin-embedded histology of 10 human fetuses at 10-16 weeks of gestation with an aid of immunohistochemistry of the intermediate filaments, matrix components (collagen types I and II, aggrecan, versican, fibronectin, tenascin, and hyaluronan) and CA9, we observed all joints and most of the entheses in the body. At any stages examined, CA9-poisitive cells were seen in the intervertebral disk and all joint cartilages including those of the facet joint of the vertebral column, but the accumulation area was reduced in the larger specimens. Glial fibrillary acidic protein (GFAP), one of the intermediate filaments, expressed in a part of the CA9-positive cartilages. Developing elastic cartilages were positive both of CA9 and GFAP. Notably, parts of the tendon or ligament facing to the joint, such as the joint surface of the annular ligament of the radius, were also positive for CA9. A distribution of each matrix components examined was not same as CA9. The bone-tendon and bone-ligament interface expressed CA9, but the duration at a site was limited to 3-4 weeks because the positive site was changed between stages. Thus, in the fetal entheses, CA9 expression displayed highly stage-dependent and site-dependent manners. CA9 in the fetal entheses seemed to play an additional role, but it was most likely to be useful as an excellent marker of mechanical stress at the start of enthesis development.
MeSH Terms
-
Aggrecans
Anoxia
Carbon*
Carbonic Anhydrases*
Cartilage
Elastic Cartilage
Fetal Development*
Fetus
Fibronectins
Glial Fibrillary Acidic Protein
Humans*
Hydrogen-Ion Concentration
Immunohistochemistry
Intermediate Filaments
Intervertebral Disc
Joints*
Ligaments*
Pregnancy
Radius
Spine
Stress, Mechanical*
Tenascin
Tendons*
Versicans
Zygapophyseal Joint
Aggrecans
Carbon
Carbonic Anhydrases
Fibronectins
Glial Fibrillary Acidic Protein
Tenascin
Versicans
Figure
Reference
-
1. Rot-Nikcevic I, Reddy T, Downing KJ, Belliveau AC, Hallgrímsson B, Hall BK, Kablar B. Myf5-/- :MyoD-/- amyogenic fetuses reveal the importance of early contraction and static loading by striated muscle in mouse skeletogenesis. Dev Genes Evol. 2006; 216:1–9.2. Mackey AL, Heinemeier KM, Koskinen SO, Kjaer M. Dynamic adaptation of tendon and muscle connective tissue to mechanical loading. Connect Tissue Res. 2008; 49:165–168.3. Kjaer M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, Doessing S, Kongsgaard M, Krogsgaard MR, Magnusson SP. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports. 2009; 19:500–510.4. Nowlan NC, Bourdon C, Dumas G, Tajbakhsh S, Prendergast PJ, Murphy P. Developing bones are differentially affected by compromised skeletal muscle formation. Bone. 2010; 46:1275–1285.5. Milz S, Benjamin M, Putz R. Molecular parameters indicating adaptation to mechanical stress in fibrous connective tissue. Adv Anat Embryol Cell Biol. 2005; 178:1–71.6. Magara J, Nozawa-Inoue K, Suzuki A, Kawano Y, Ono K, Nomura S, Maeda T. Alterations in intermediate filaments expression in disc cells from the rat temporomandibular joint following exposure to continuous compressive force. J Anat. 2012; 220:612–621.7. Abe S, Rhee SK, Osonoi M, Nakamura T, Cho BH, Murakami G, Ide Y. Expression of intermediate filaments at muscle insertions in human fetuses. J Anat. 2010; 217:167–173.8. Yang Y, Makita T. Immunocytochemical colocalization of desmin and vimentin in human fetal skeletal muscle cells. Anat Rec. 1996; 246:64–70.9. Kepes JJ, Perentes E. Glial fibrillary acidic protein in chondrocytes of elastic cartilage in the human epiglottis: an immunohistochemical study with polyvalent and monoclonal antibodies. Anat Rec. 1988; 220:296–299.10. Viale G, Doglioni C, Dell'Orto P, Zanetti G, Iuzzolino P, Bontempini L, Coggi G. Glial fibrillary acidic protein immunoreactivity in human respiratory tract cartilages and pulmonary chondromatous hamartomas. Am J Pathol. 1988; 133:363–373.11. Supuran CT. Carbonic anhydrases: an overview. Curr Pharm Des. 2008; 14:603–614.12. Hilvo M, Innocenti A, Monti SM, De Simone G, Supuran CT, Parkkila S. Recent advances in research on the most novel carbonic anhydrases, CA XIII and XV. Curr Pharm Des. 2008; 14:672–678.13. Gilmour KM. Perspectives on carbonic anhydrase. Comp Biochem Physiol A Mol Integr Physiol. 2010; 157:193–197.14. Takacova M, Barathova M, Hulikova A, Ohradanova A, Kopacek J, Parkkila S, Pastorek J, Pastorekova S, Zatovicova M. Hypoxia-inducible expression of the mouse carbonic anhydrase IX demonstrated by new monoclonal antibodies. Int J Oncol. 2007; 31:1103–1110.15. Barathova M, Takacova M, Holotnakova T, Gibadulinova A, Ohradanova A, Zatovicova M, Hulikova A, Kopacek J, Parkkila S, Supuran CT, Pastorekova S, Pastorek J. Alternative splicing variant of the hypoxia marker carbonic anhydrase IX expressed independently of hypoxia and tumour phenotype. Br J Cancer. 2008; 98:129–136.16. Schultz M, Jin W, Waheed A, Moed BR, Sly W, Zhang Z. Expression profile of carbonic anhydrases in articular cartilage. Histochem Cell Biol. 2011; 136:145–151.17. Liao SY, Lerman MI, Stanbridge EJ. Expression of transmembrane carbonic anhydrases, CAIX and CAXII, in human development. BMC Dev Biol. 2009; 9:22.18. Scheibe RJ, Mundhenk K, Becker T, Hallerdei J, Waheed A, Shah GN, Sly WS, Gros G, Wetzel P. Carbonic anhydrases IV and IX: subcellular localization and functional role in mouse skeletal muscle. Am J Physiol Cell Physiol. 2008; 294:C402–C412.19. Gut MO, Parkkila S, Vernerová Z, Rohde E, Závada J, Höcker M, Pastorek J, Karttunen T, Gibadulinová A, Závadová Z, Knobeloch KP, Wiedenmann B, Svoboda J, Horak I, Pastoreková S. Gastric hyperplasia in mice with targeted disruption of the carbonic anhydrase gene Car9. Gastroenterology. 2002; 123:1889–1903.20. Hayashi S, Murakami G, Ohtsuka A, Itoh M, Nakano T, Fukuzawa Y. Connective tissue configuration in the human liver hilar region with special reference to the liver capsule and vascular sheath. J Hepatobiliary Pancreat Surg. 2008; 15:640–647.21. Kawase T, Shibata S, Katori Y, Ohtsuka A, Murakami G, Fujimiya M. Elastic fiber-mediated enthesis in the human middle ear. J Anat. 2012; 221:331–340.22. Shibata S, Fukada K, Imai H, Abe T, Yamashita Y. In situ hybridization and immunohistochemistry of versican, aggrecan and link protein, and histochemistry of hyaluronan in the developing mouse limb bud cartilage. J Anat. 2003; 203:425–432.23. Nakasone A, Shibata S, Suzuki S, Yamashita Y, Ohyama K. Laser burn wound healing in naso-labial region of fetal and neonatal mice. Oral Dis. 2007; 13:45–50.24. Yokohama-Tamaki T, Maeda T, Tanaka TS, Shibata S. Functional analysis of CTRP3/cartducin in Meckel's cartilage and developing condylar cartilage in the fetal mouse mandible. J Anat. 2011; 218:517–533.25. Palmer AK. Triangular fibrocartilage complex lesions: a classification. J Hand Surg Am. 1989; 14:594–606.26. Nam YS, Han SH, Shin SY. Detailed anatomy of the capsulopalpebral fascia. Clin Anat. 2012; 25:709–713.27. Provot S, Zinyk D, Gunes Y, Kathri R, Le Q, Kronenberg HM, Johnson RS, Longaker MT, Giaccia AJ, Schipani E. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol. 2007; 177:451–464.28. Miyako H, Suzuki A, Nozawa-Inoue K, Magara J, Kawano Y, Ono K, Maeda T. Phenotypes of articular disc cells in the rat temporomandibular joint as demonstrated by immunohistochemistry for nestin and GFAP. J Anat. 2011; 219:472–480.29. Katori Y, Takeuchi H, Rodríguez-Vázquez JF, Kitano H, Murakami G, Kawase T. Fetal development of the human epiglottis revisited: appearance of GFAP-positive mesenchymal cells and fibrous connections with other laryngeal and lingual structures. Ann Anat. 2011; 193:149–155.30. Ikari Y, Katori Y, Ohtsuka A, Rodríguez-Vázquez JF, Abe H, Kawase T, Murakami G, Abe S. Fetal development and variations in the cartilages surrounding the human external acoustic meatus. Ann Anat. 2013; 195:128–136.31. Kinoshita H, Umezawa T, Omine Y, Kasahara M, Rodríguez-Vázquez JF, Murakami G, Abe S. Distribution of elastic fibers in the head and neck: a histological study using late-stage human fetuses. Anat Cell Biol. 2013; 46:39–48.32. Watanabe H, Miake K, Sasaki J. Immunohistochemical study of the cytoskeleton of osteoblasts in the rat calvaria. Intermediate filaments and microfilaments as demonstrated by detergent perfusion. Acta Anat (Basel). 1993; 147:14–23.33. Shapiro F, Cahill C, Malatantis G, Nayak RC. Transmission electron microscopic demonstration of vimentin in rat osteoblast and osteocyte cell bodies and processes using the immunogold technique. Anat Rec. 1995; 241:39–48.34. Abe S, Nakamura T, Rodriguez-Vazquez JF, Murakami G, Ide Y. Early fetal development of the rotator interval region of the shoulder with special reference to topographical relationships among related tendons and ligaments. Surg Radiol Anat. 2011; 33:609–615.35. Abe S, Suzuki M, Cho KH, Murakami G, Cho BH, Ide Y. CD34-positive developing vessels and other structures in human fetuses: an immunohistochemical study. Surg Radiol Anat. 2011; 33:919–927.36. Berger RA, Kauer JM, Landsmeer JM. Radioscapholunate ligament: a gross anatomic and histologic study of fetal and adult wrists. J Hand Surg Am. 1991; 16:350–355.37. Mérida-Velasco JA, Garcia-Garcia JD, Espín-Ferra J, Sánchez-Montesinos I. Development of the human wrist joint ligaments. Anat Rec. 1996; 245:114–121.38. Yang JD, Hwang HP, Kim JH, Rodríguez-Vázquez JF, Abe S, Murakami G, Cho BH. Development of the rectus abdominis and its sheath in the human fetus. Yonsei Med J. 2012; 53:1028–1035.39. Cho KH, Kim JH, Ha YS, Murakami G, Cho BH, Abe S. Development of the deep flexor tendons and lumbricalis muscle in the hand and foot: a histological study using human mid-term foetuses. Folia Morphol (Warsz). 2012; 71:154–163.40. Väänänen HK. Immunohistochemical localization of carbonic anhydrase isoenzymes I and II in human bone, cartilage and giant cell tumor. Histochemistry. 1984; 81:485–487.41. Osanai H, Rodríguez-Vázquez JF, Abe H, Murakami G, Ohguro H, Fujimiya M. Fetal check ligament connected between the conjunctiva and the medial and lateral recti. Invest Ophthalmol Vis Sci. 2011; 52:7175–7179.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Topical Carbonic Anhydrase Inhibitors on Nitric Oxide Production in Trabecular Meshwork Cells
- Carbonic Anhyd rase Activity in Muller Cell
- The Expression of Carbonic Anhydrase Isozymes in Human Intestine
- Effects of carbonic anhydrase inhibitors on the lps-induced bone resorption in vitro
- Histochemical study on trematodes - Distribution of carbonic anhydrase activity