Anat Cell Biol.
2012 Sep;45(3):178-184. 10.5115/acb.2012.45.3.178.
Effects of high-fat diet on the numerical density and number of neuronal cells and the volume of the mouse hypothalamus: a stereological study
- Affiliations
-
- 1Histomorphometry and Stereology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. namavarreza@yahoo.com
- 2Department of Anatomical Sciences, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
- KMID: 2046745
- DOI: http://doi.org/10.5115/acb.2012.45.3.178
Abstract
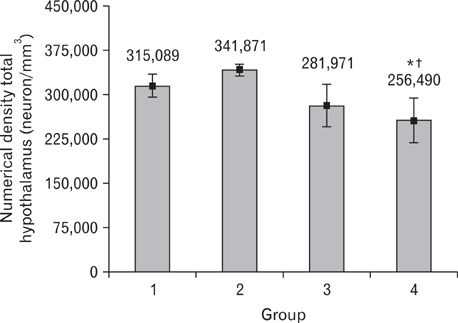
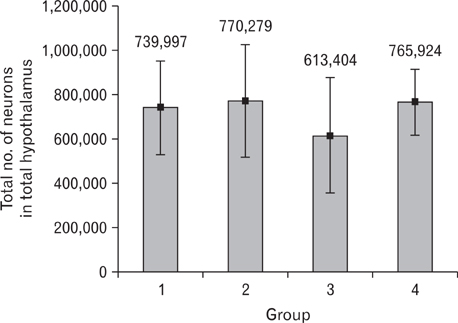
- It has been demonstrated that the type of diet affects the brain structure and function. Consumption of fat-rich food is one of the most important factors that lead to increase in the prevalence of cardiovascular and neurological diseases. High-fat diet may change the volume and neuronal number or density in the hypothalamus, which is the center of energy control. Therefore, this study was designed to study the effect of high-fat diet on the density and number of neurons, and also the volume of hypothalamus in adult male mice. Forty male mice were divided into the control and experimental groups. The control group were fed with standard and the experimental groups, with high-fat diet for 4 (short-term) or 8 (long-term) weeks. The animals were perfused and brains were immediately removed, post-fixed and cut coronally and serially using cryostat at 30-microm thickness. Every 6th sections were stained by cresyl violet. The numerical density and number of neuron and the volume of hypothalamus were estimated by using unbiased stereological methods. Data analysis showed that both short and long time consumption of high-fat diet decreased the neuronal cell density of the hypothalamus. Interestingly, despite a decrease in the neuronal cell density, long time consumption of high-fat diet could significantly increase the volume of hypothalamus (P<0.05). High fat diet decreased the neuronal cell density and increased the volume of the hypothalamus, but it did not significantly change its total neurons. These changes might be due to an increase in the extracellular space through inflammation or gliosis in the hypothalamus.
Keyword
MeSH Terms
Figure
Reference
-
1. Formiguera X, Cantón A. Obesity: epidemiology and clinical aspects. Best Pract Res Clin Gastroenterol. 2004. 18:1125–1146.2. Townsend KL. Mechanisms of high fat induced-obesity in mice and premigration/prehibernation fattening in rats, in graduate school of arts and sciences. 2008. Boston: Boston University;170–172.3. Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira AL, Saad MJ, Velloso LA. High-fat diet induces apoptosis of hypothalamic neurons. PLoS One. 2009. 4:e5045.4. Rada P, Bocarsly ME, Barson JR, Hoebel BG, Leibowitz SF. Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiol Behav. 2010. 101:394–400.5. Cammisotto P, Bendayan M. A review on gastric leptin: the exocrine secretion of a gastric hormone. Anat Cell Biol. 2012. 45:1–16.6. Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Schölmerich J, Bollheimer LC. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol. 2006. 36:485–501.7. Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010. 219:25–32.8. De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005. 146:4192–4199.9. Yi CO, Jeon BT, Shin HJ, Jeong EA, Chang KC, Lee JE, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Resveratrol activates AMPK and suppresses LPS-induced NF-kappaB-dependent COX-2 activation in RAW 264.7 macrophage cells. Anat Cell Biol. 2011. 44:194–203.10. Wang C, Godar RJ, Billington CJ, Kotz CM. Chronic administration of brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reverses obesity induced by high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2010. 298:R1320–R1332.11. Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2003. 24:225–253.12. Townsend KL, Lorenzi MM, Widmaier EP. High-fat diet-induced changes in body mass and hypothalamic gene expression in wild-type and leptin-deficient mice. Endocrine. 2008. 33:176–188.13. Unger TJ. Examination of deficits in energy balance and affective behavior following central or hypothalamic depletion of brain-derived neurotrophic factor. 2007. Boston: Sackler School of Graduate Biomedical Sciences (Tufts University);194.14. Howard CV, Reed MG. Unbiased stereology: three-dimensional measurement in microscopy. 1998. Oxford: Bios Scientific Publisher.15. Mouton PR. Principles and practices of unbiased stereology: an introduction for bioscientists. 2002. Baltimore: Johns Hopkins University Press.16. Abusaad I, MacKay D, Zhao J, Stanford P, Collier DA, Everall IP. Stereological estimation of the total number of neurons in the murine hippocampus using the optical disector. J Comp Neurol. 1999. 408:560–566.17. Gundersen HJ, Jensen EB, Kiêu K, Nielsen J. The efficiency of systematic sampling in stereology: reconsidered. J Microsc. 1999. 193(Pt 3):199–211.18. Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry. 2001. 50:395–406.19. Korbo L, Amrein I, Lipp HP, Wolfer D, Regeur L, Oster S, Pakkenberg B. No evidence for loss of hippocampal neurons in non-Alzheimer dementia patients. Acta Neurol Scand. 2004. 109:132–139.20. Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. 2001. 2nd ed. San Diego: Academic Press.21. De Souza CT, Pereira-da-Silva M, Araujo EP, Morari J, Alvarez-Rojas F, Bordin S, Moreira-Filho DC, Carvalheira JB, Saad MJ, Velloso LA. Distinct subsets of hypothalamic genes are modulated by two different thermogenesis-inducing stimuli. Obesity (Silver Spring). 2008. 16:1239–1247.22. Park ES, Yi SJ, Kim JS, Lee HS, Lee IS, Seong JK, Jin HK, Yoon YS. Changes in orexin-A and neuropeptide Y expression in the hypothalamus of the fasted and high-fat diet fed rats. J Vet Sci. 2004. 5:295–302.23. Pirnik Z, Bundzikova J, Mikkelsen JD, Zelezna B, Maletinska L, Kiss A. Fos expression in hypocretinergic neurons in C57B1/6 male and female mice after long-term consumption of high fat diet. Endocr Regul. 2008. 42:137–146.24. Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009. 132(Pt 4):889–902.25. Amin KA, Kamel HH, Abd Eltawab MA. The relation of high fat diet, metabolic disturbances and brain oxidative dysfunction: modulation by hydroxy citric acid. Lipids Health Dis. 2011. 10:74.26. Valladolid-Acebes I, Stucchi P, Cano V, Fernández-Alfonso MS, Merino B, Gil-Ortega M, Fole A, Morales L, Ruiz-Gayo M, Del Olmo N. High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiol Learn Mem. 2011. 95:80–85.27. Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis. 2008. 14:133–145.28. Liu M, Shen L, Liu Y, Woods SC, Seeley RJ, D'Alessio D, Tso P. Obesity induced by a high-fat diet downregulates apolipoprotein A-IV gene expression in rat hypothalamus. Am J Physiol Endocrinol Metab. 2004. 287:E366–E370.29. Regeur L. Increasing loss of brain tissue with increasing dementia: a stereological study of post-mortem brains from elderly females. Eur J Neurol. 2000. 7:47–54.30. Young JK. A comparison of hypothalami of rats and mice: lack of gross sexual dimorphism in the mouse. Brain Res. 1982. 239:233–239.31. Langdon KD, Clarke J, Corbett D. Long-term exposure to high fat diet is bad for your brain: exacerbation of focal ischemic brain injury. Neuroscience. 2011. 182:82–87.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulating Hypothalamus Gene Expression in Food Intake: Dietary Composition or Calorie Density?
- Changes in Neuropeptide Y-Immunoreactive Cells in the Hypothalamus and Cajal Interstitial Cells in the Small Intestine of Rats with High-Fat Diet
- Effects of a Low-carbohydrate, High-fat Diet
- Effect of high-fat diet on the various morphological parameters of the ovary
- Alteration in plasma chemokine profile in a high-fat diet-induced obesity mouse model