Lab Med Online.
2015 Oct;5(4):211-214. 10.3343/lmo.2015.5.4.211.
Evaluation of the Urinary Glucose Tetrasaccharide Assay Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry for Diagnosis of Pompe Disease
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea. songjhcp@snu.ac.kr
- 2Department of Laboratory Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Laboratory Medicine & Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2046383
- DOI: http://doi.org/10.3343/lmo.2015.5.4.211
Abstract
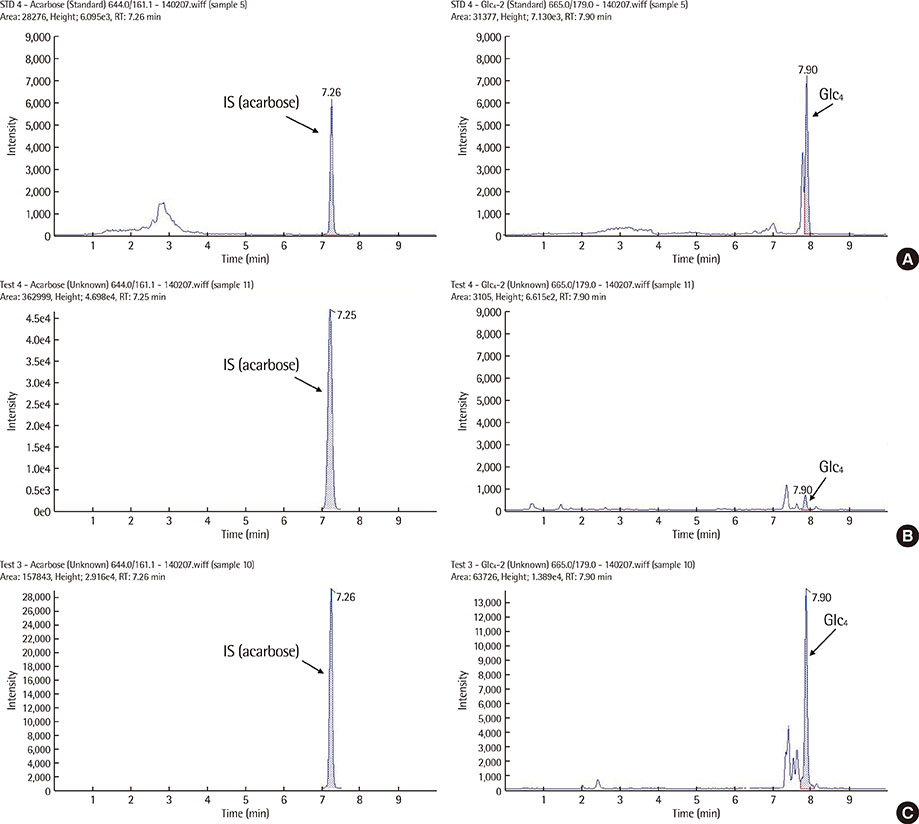
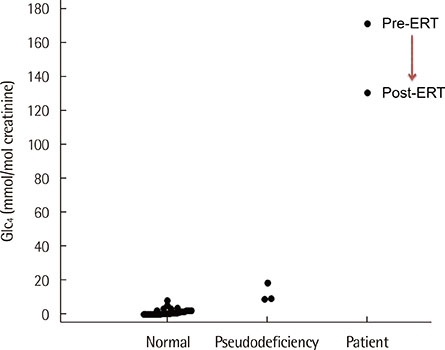
- We evaluated the urinary glucose tetrasaccharide (Glc4) assay using ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). The calibration curve was linear over a range of 5-500 micromol/L. Performance parameters such as intra- and inter-day imprecision CVs were 6.52-14.6% and 11.5-13.2%, respectively. The mean concentrations of urinary Glc4 in 27 normal controls and 3 pseudodeficiency patients were 1.5 and 12.1 mmol/mol creatinine, respectively. Urinary Glc4 concentration in a patient with Pompe disease was 171.3 mmol/mol creatinine, which decreased to 130.9 mmol/mol following enzyme replacement therapy. Based on our results, we suggest that the urinary Glc4 assay using UPLC-MS/MS can be a reliable diagnostic tool for identification of patients with Pompe disease.
MeSH Terms
Figure
Reference
-
1. van der Ploeg AT, Reuser AJ. Pompe's disease. Lancet. 2008; 372:1342–1353.
Article2. Cho A, Jeong GU, Lim BC, Park JY, Moon JH, Chae JH, et al. Clinical characteristics of childhood Pompe disease. J Korean Child Neurol Soc. 2007; 15:83–89.3. Manwaring V, Prunty H, Bainbridge K, Burke D, Finnegan N, Franses R, et al. Urine analysis of glucose tetrasaccharide by HPLC; a useful marker for the investigation of patients with Pompe and other glycogen storage diseases. J Inherit Metab Dis. 2012; 35:311–316.
Article4. Winchester B, Bali D, Bodamer OA, Caillaud C, Christensen E, Cooper A, et al. Methods for a prompt and reliable laboratory diagnosis of Pompe disease: report from an international consensus meeting. Mol Genet Metab. 2008; 93:275–281.
Article5. Labrousse P, Chien YH, Pomponio RJ, Keutzer J, Lee NC, Akmaev VR, et al. Genetic heterozygosity and pseudodeficiency in the Pompe disease newborn screening pilot program. Mol Genet Metab. 2010; 99:379–383.
Article6. Oda E, Tanaka T, Migita O, Kosuga M, Fukushi M, Okumiya T, et al. Newborn screening for Pompe disease in Japan. Mol Genet Metab. 2011; 104:560–565.
Article7. Kumamoto S, Katafuchi T, Nakamura K, Endo F, Oda E, Okuyama T, et al. High frequency of acid alpha-glucosidase pseudodeficiency complicates newborn screening for glycogen storage disease type II in the Japanese population. Mol Genet Metab. 2009; 97:190–195.
Article8. Chiang SC, Hwu WL, Lee NC, Hsu LW, Chien YH. Algorithm for Pompe disease newborn screening: results from the Taiwan screening program. Mol Genet Meta. 2012; 106:281–286.
Article9. Young SP, Stevens RD, An Y, Chen YT, Millington DS. Analysis of a glucose tetrasaccharide elevated in Pompe disease by stable isotope dilution–electrospray ionization tandem mass spectrometry. Anal Biochem. 2003; 316:175–180.
Article10. Young SP, Piraud M, Goldstein JL, Zhang H, Rehder C, Laforet P, et al. Assessing disease severity in Pompe disease: the roles of a urinary glucose tetrasaccharide biomarker and imaging techniques. Am J Med Genet C Semin Med Genet. 2012; 160C:50–58.
Article11. Lennartson G, Lundblad A, Sjöblad S, Svensson S, Ockerman PA. Quantitation of a urinary tetrasaccharide by gas chromatography and mass spectrometry. Biomed Mass Spectrom. 1976; 3:51–54.
Article12. An Y, Young SP, Kishnani PS, Millington DS, Amalfitano A, Corz D, et al. Glucose tetrasaccharide as a biomarker for monitoring the therapeutic response to enzyme replacement therapy for Pompe disease. Mol Genet Metab. 2005; 85:247–254.
Article13. An Y, Young SP, Hillman SL, Van Hove JL, Chen YT, Millington DS. Liquid chromatographic assay for a glucose tetrasaccharide, a putative biomarker for the diagnosis of Pompe disease. Anal Biochem. 2000; 287:136–143.
Article14. Sluiter W, van den Bosch JC, Goudriaan DA, van Gelder CM, de Vries JM, Huijmans JG, et al. Rapid ultraperformance liquid chromatography–tandem mass spectrometry assay for a characteristic glycogen-derived tetrasaccharide in Pompe disease and other glycogen storage diseases. Clin Chem. 2012; 58:1139–1147.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of Elecsys Vitamin D Total II Assay Using Roche Modular Analytics E170
- Simultaneous Screening of 177 Drugs of Abuse in Urine Using Ultra-performance Liquid Chromatography with Tandem Mass Spectrometry in Drug-intoxicated Patients
- Identification of Novel Metabolic Proteins Released by Insulin Signaling of the Rat Hypothalmus Using Liquid Chromatography-Mass Spectrometry (LC-MS)
- Implementation and Validation of an Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry Method for Quantifying Levetiracetam and Lamotrigine in Serum Specimens
- Metabolism and excretion of novel pulmonary-targeting docetaxel liposome in rabbits