J Adv Prosthodont.
2009 Jul;1(2):91-96. 10.4047/jap.2009.1.2.91.
Implant surface treatments affect gene expression of Runx2, osteogenic key marker
- Affiliations
-
- 1Department of Dental Prosthodontics, Seoul National University College of Dentistry, Seoul, Korea. young21c@snu.ac.kr
- KMID: 2045469
- DOI: http://doi.org/10.4047/jap.2009.1.2.91
Abstract
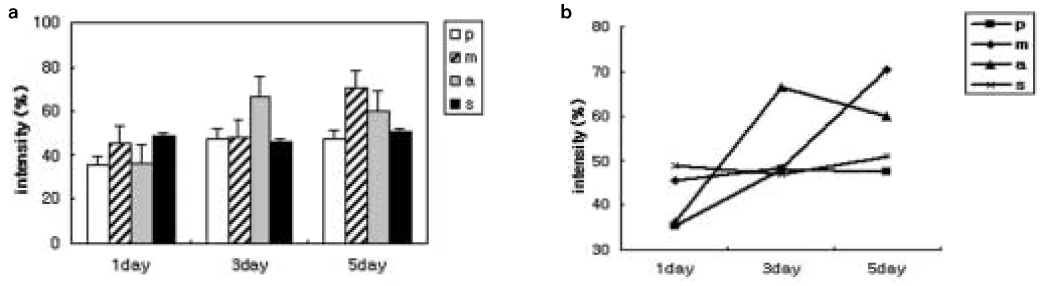
- STATEMENT OF PROBLEM: The aim of this study was to study the effects of various surface treatments to a titanium surface on the expression of Runx2 in vitro. MATERIAL AND METHODS: Human Osteosarcoma TE-85 cells were cultured on machined, sandblasted, or anodic oxidized cpTi discs. At various times of incubation, the cells were collected and then processed for the analysis of mRNA expression of Runx2 using reverse transcription-PCR. RESULTS: The expression pattern of Runx2 mRNA was differed according to the types of surface treatment. When the cells were cultured on the untreated control culture plates, the gene expression of Runx2 was not increased during the experiments. In the case of that the cells were cultured on the machined cpTI discs, the expression level was intermediate at the first day, but increased constitutively to day 5. In cells on sandblasted cpTi discs, the expression level was highest in the first day sample and the level was maintained to 5 days. In cells on anodized cpTi discs, the expression level increased rapidly to 3 days, but decreased slightly in the 5-th day sample. CONCLUSION: Different surface treatments may contribute to the regulation of osteoblast function by influencing the level of gene expression of key osteogenic factors.
MeSH Terms
Figure
Reference
-
1. Davies JE. Understanding peri-implant endosseous healing. J Dent Educ. 2003. 67:932–949.2. Schwartz Z, Kieswetter K, Dean DD, Boyan BD. Underlying mechanisms at the bone-surface interface during regeneration. J Periodontal Res. 1997. 32:166–171.3. Wennerberg A. Implant design and surface factors. Int J prosthodont. 2003. 16:45–51.4. Bigerelle M, Anselme K, Noel B, Ruderman I, Hardouin P, Iost A. Improvement in the morphology of Ti-based surfaces: a new process to increase in vitro human osteoblast response. Biomaterials. 2002. 23:1563–1577.5. Ratner BD. Replacing and renewing: synthetic materials, biomimetics, and tissue engineering in implant dentistry. J Dent Educ. 2001. 65:1340–1349.6. Li LH, Kong YM, Kim YW, Kim HE, Heo SJ, Koak JY. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials. 2004. 25:2867–2875.7. Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulation bone and cartilage cell response. Biomaterials. 1996. 17:137–146.8. Xavier SP, Carvalho PS, Beloti MM, Rosa AL. Response of rat bone marrow cells to commercially pure titanium submitted to different surface treatments. J Dent. 2003. 31:173–180.9. Bowers KT, Keller JC, Randolph BA, Wick DG, Michaels CM. Optimization of surface micromorphology for enhanced osteoblast responses in vitro. Int J Oral Maxillofac Implants. 1992. 7:302–310.10. Ong JL, Prince CW, Raikar GN, Lucas LC. Effect of surface topography of titanium on surface chemistry and cellular response. Implant Dent. 1996. 5:83–88.11. Schneider G, Burridge K. Formation of focal adhesions by osteoblasts adhering to different substrata. Exp Cell Res. 1994. 214:264–269.12. Masuda T, Salvi GE, Offenbacher S, Felton DA, Cooper LE. Cell and matrix reactions at titanium implants in surgically prepared rat tibiae. Int J Oral Maxillofac Implants. 1997. 12:472–485.13. Schwartz Z, Lohmann CH, Oefinger J, Bonewald LF, Dean DD, Boyan BD. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv Dent Res. 1999. 13:38–48.14. Carinci F, Pezzetti F, Volinia S, Francioso F, Arcelli D, Marchesini J, Scapoli L, Piattelli A. Analysis of osteoblast-like MG63 cells' response to a rough implant surface by means of DNA microarray. J Oral Implantol. 2003. 29:215–220.15. Schneider GBH, Perinpanayagam H, Clegg M, Zaharias R, Seabold D, Keller J, Stanford C. Implant surface roughness affects osteoblast gene expression. J Dent Res. 2003. 82:372–377.16. Schneider GB, Zaharias R, Seabold D, Keller J, Stanford C. Differentiation of preosteoblasts is affected by implant surface microtopographies. J Biomed Mater Res A. 2004. 69:462–468.17. Ogawa T, Sukotjo C, Nishimura I. Modulated bone matrix-related gene expression is associated with differences in interfacial strength of different implant surface roughness. J Prosthodont. 2002. 11:241–247.18. Shui C, Spelsberg TC, Riggs BL, Khosla S. Changes in Runx2/Cbfa1 expression and activity during osteoblastic differentiation of human bone marrow stromal cells. J Bone Miner Res. 2003. 18:213–221.19. Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RJ. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the osf2 transcription factor. J Biol Chem. 1998. 273:32988–32994.20. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997. 89:747–754.21. Komori T. A fundamental transcription factor for bone and cartilage. Biochem Biophys Res Commun. 2000. 276:813–816.22. Komori T. Runx2, A multifunctional transcription factor in skeletal development. J Cell biochem. 2002. 87:1–8.23. Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 1999. 13:1025–1036.24. Prince M, Banerjee C, Javed A, Green J, Lian JB, Stein GS, Bodine PV, Komm BS. Expression and regulation of Runx2/Cbfa1 and osteoblast phenotypic markers during the growth and differentiation of human osteoblasts. J Cell Biochem. 2001. 80:424–440.25. Ducy P. Cbfa1: a molecular switch in osteoblast biology. Dev Dyn. 2000. 219:461–471.26. Schneider GB, Zaharias R, Stanford C. Osteoblast integrin adhesion and signaling regulate mineralization. J Dent Res. 2001. 80:1540–1544.27. Byers BA, Pavlath GK, Murphy TJ, Karsenty G, Garcia AJ. Cell-type-dependent up-regulation of in vitro mineralization after overexpression of the osteoblast-specific transcription factor Runx2/Cbfal. J Bone Miner Res. 2002. 17:1931–1944.28. Brett PM, Harle J, Salih V, Mihoc R, Olsen I, Jones FH, Tonetti M. Roughness response genes in osteoblasts. Bone. 2004. 35:124–133.29. Ziros PG, Gil AP, Georgakopoulos T, Habeos I, Kletsas D, Basdra EK, Papavassiliou AG. The bone-specific transcriptional regulator Cbfa1 is a target of mechanical signals in osteoblastic cells. J Biol Chem. 2002. 277:23934–23941.30. Sykaras N, Iacopino AM, Marker VA, Triplett RG, Woody RD. Implant materials, designs, and surface topographies: their effect on osseointegration. Int J Oral Maxillofac Implants. 2000. 15:675–690.31. Sinha RK, Tuan RS. Regulation of human osteoblast integrin expression by orthopedic implant materials. Bone. 1996. 18:451–457.32. Puleo DA, Holleran LA, Doremus RH, Bizios R. Osteoblast responses to orthopedic implant materials in vitro. J Biomed Mater Res. 1991. 25:711–723.33. Franceschi RT. The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit Rev Oral Biol Med. 1999. 10:40–57.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoxia Enhances Osteogenic Differentiation in Retinoic Acid-Treated Murine-Induced Pluripotent Stem Cells
- Zinc upregulates bone-specific transcription factor Runx2 expression via BMP-2 signaling and Smad-1 phosphorylation in osteoblasts
- CA1 Modulates the Osteogenic Differentiation of Dental Follicle Stem Cells by Activating the BMP Signaling Pathway In Vitro
- Expression of RUNX2/LAPTM5 in the Induction of MC3T3-e1 Mineralization and Its Possible Relationship with Autophagy
- YBX1 Promotes the Inclusion of RUNX2 Alternative Exon 5 in Dental Pulp Stem Cells