Protective Effects of KH-204 in the Bladder of Androgen-Deprived Rats
- Affiliations
-
- 1Catholic Integrative Medicine Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Department of Urology, College of Medicine, The Catholic University of Korea, Seoul, Korea. ksw1227@catholic.ac.kr
- 3Department of Urology, Second Hospital of Lanzhou University, Lanzhou, China.
- 4Korea Bio Medical Science Institute, Seoul, Korea.
- KMID: 2032826
- DOI: http://doi.org/10.5534/wjmh.2015.33.2.73
Abstract
- PURPOSE
We investigated the protective effects of the herbal formulation KH-204 in the bladder of androgen-deprived rats.
MATERIALS AND METHODS
Male rats aged eight weeks were randomly divided into four groups, containing eight rats each: sham operation only (normal control group), androgen-deprived only (androgen-deprived control group), and androgen-deprived followed by treatment with 200 mg/kg or 400 mg/kg of KH-204. After 0.5 mg/kg of leuprorelin was subcutaneously injected in the androgen-deprived groups, the oral administration of either distilled water in the two control groups or KH-204 in the treatment group was continued for four weeks. Serum testosterone levels, RhoGEF levels, nitric oxide (NO)-cyclic guanosine monophosphate (cGMP)-related parameters, oxidative stress, and histologic changes were evaluated after treatment.
RESULTS
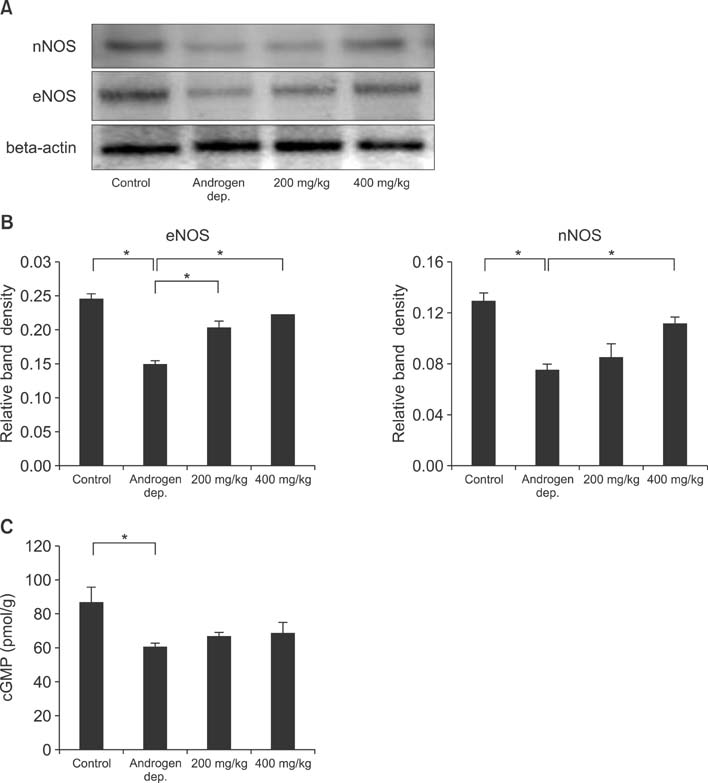
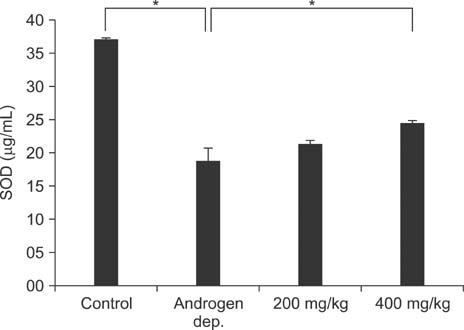
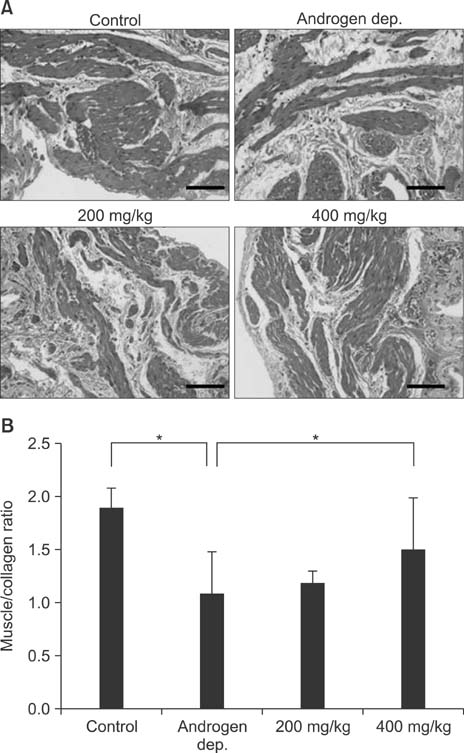
Treatment with the herbal formulation KH-204 (1) increased serum testosterone levels; (2) restored the expression of RhoGEFs, endothelial NO synthase, and neuronal NO synthase; (3) increased the expression of superoxide dismutase; and (4) decreased bladder fibrosis.
CONCLUSIONS
Our results suggest that the positive effects of KH-204 on the urinary bladder may be attributed to its antioxidant effects or to an elevation in NO-cGMP activity.
Keyword
MeSH Terms
-
Administration, Oral
Animals
Antioxidants
Fibrosis
Guanosine Monophosphate
Humans
Hypogonadism
Leuprolide
Male
Neurons
Nitric Oxide
Nitric Oxide Synthase
Oxidative Stress
Phytotherapy
Rats*
Rho Guanine Nucleotide Exchange Factors
Superoxide Dismutase
Testosterone
Urinary Bladder*
Water
Antioxidants
Guanosine Monophosphate
Leuprolide
Nitric Oxide
Nitric Oxide Synthase
Superoxide Dismutase
Testosterone
Water
Figure
Cited by 4 articles
-
Synergistic effects of extracorporeal shockwave therapy and modified Ojayeonjonghwan on erectile dysfunction in an animal model of diabetes
Hyun Cheol Jeong, Woong Jin Bae, Guan Qun Zhu, Seung Hwan Jeon, Sae Woong Choi, Su Jin Kim, Hyuk Jin Cho, Sung-Hoo Hong, Ji Youl Lee, Sung Yeoun Hwang, Sae Woong Kim
Investig Clin Urol. 2019;60(4):285-294. doi: 10.4111/icu.2019.60.4.285.Development of an Improved Animal Model of Overactive Bladder: Transperineal Ligation
versus Transperitoneal Ligation in Male Rats
Woo Hyun Kim, Woong Jin Bae, Jung Woo Park, Jin Bong Choi, Su Jin Kim, Hyuk Jin Cho, U Syn Ha, Sung Hoo Hong, Ji Youl Lee, Sung Yeoun Hwang, Sae Woong Kim
World J Mens Health. 2016;34(2):137-144. doi: 10.5534/wjmh.2016.34.2.137.Administration of Goji (
Lycium chinense Mill.) Extracts Improves Erectile Function in Old Aged Rat Model
Hyong Woo Moon, Jung Woo Park, Kyu Won Lee, Hyun Cheol Jeong, Jin Bong Choi, Sae Woong Choi, Woong Jin Bae, Hyuk Jin Cho, U-Syn Ha, Sung Hoo Hong, Jeong Ho Geum, Seong Bin Hong, Sae Woong Kim
World J Mens Health. 2017;35(1):43-50. doi: 10.5534/wjmh.2017.35.1.43.Effect of Korean Herbal Formula (Modified Ojayeonjonghwan) on Androgen Receptor Expression in an Aging Rat Model of Late Onset Hypogonadism
Sae Woong Choi, Seung Hwan Jeon, Eun Bi Kwon, Guan Qun Zhu, Kyu Won Lee, Jin Bong Choi, Hyun Cheol Jeong, Kang Sup Kim, Sang Rak Bae, Woong Jin Bae, Su Jin Kim, Hyuk Jin Cho, U-Syn Ha, Sung-Hoo Hong, Sung Yeoun Hwang, Sae Woong Kim
World J Mens Health. 2019;37(1):105-112. doi: 10.5534/wjmh.180051.
Reference
-
1. Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, et al. International Society of Andrology. International Society for the Study of Aging Male. European Association of Urology. European Academy of Andrology. American Society of Andrology. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. Eur Urol. 2009; 55:121–130.
Article2. Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010; 363:123–135.
Article3. Wu CY, Yu TJ, Chen MJ. Age related testosterone level changes and male andropause syndrome. Chang Gung Med J. 2000; 23:348–353.4. Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002; 87:589–598.
Article5. Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, et al. European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008; 93:2737–2745.
Article6. Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002; 57:M76–M99.
Article7. Shigehara K, Namiki M. Late-onset hypogonadism syndrome and lower urinary tract symptoms. Korean J Urol. 2011; 52:657–663.
Article8. Madeiro A, Girão M, Sartori M, Acquaroli R, Baracat E, Rodrigues De Lima G. Effects of the association of androgen/estrogen on the bladder and urethra of castrated rats. Clin Exp Obstet Gynecol. 2002; 29:117–120.9. Pradidarcheep W. Lower urinary tract symptoms and its potential relation with late-onset hypogonadism. Aging Male. 2008; 11:51–55.
Article10. McVary K. Lower urinary tract symptoms and sexual dysfunction: epidemiology and pathophysiology. BJU Int. 2006; 97:Suppl 2. 23–28. discussion 44-5
Article11. Gomelsky A, Dmochowski RR. Urodynamic effects of once-daily tadalafil in men with LUTS secondary to clinical BPH. Curr Urol Rep. 2010; 11:254–260.
Article12. Barqawi A, Crawford ED. Testosterone replacement therapy and the risk of prostate cancer. Is there a link? Int J Impot Res. 2006; 18:323–328.
Article13. Park CS, Ryu SD, Hwang SY. Elevation of intracavernous pressure and NO-cGMP activity by a new herbal formula in penile tissues of aged and diabetic rats. J Ethnopharmacol. 2004; 94:85–92.
Article14. Bae WJ, Ha US, Kim KS, Kim SJ, Cho HJ, Hong SH, et al. Effects of KH-204 on the expression of heat shock protein 70 and germ cell apoptosis in infertility rat models. BMC Complement Altern Med. 2014; 14:367.
Article15. Gotanda K, Shinbo A, Okada M, Nakano Y, Kobayashi H, Sasaki T, et al. Effects of combination therapy with a luteinizing hormone-releasing hormone agonist and chlormadinone acetate on rat prostate weight and plasma testosterone levels. Prostate Cancer Prostatic Dis. 2003; 6:66–72.
Article16. Troen BR. The biology of aging. Mt Sinai J Med. 2003; 70:3–22.17. Dröge W. Oxidative stress and aging. Adv Exp Med Biol. 2003; 543:191–200.
Article18. Chun AL, Wallace LJ, Gerald MC, Levin RM, Wein AJ. Effect of age on in vivo urinary bladder function in the rat. J Urol. 1988; 139:625–627.
Article19. Lluel P, Palea S, Barras M, Grandadam F, Heudes D, Bruneval P, et al. Functional and morphological modifications of the urinary bladder in aging female rats. Am J Physiol Regul Integr Comp Physiol. 2000; 278:R964–R972.20. Yassin AA, El-Sakka AI, Saad F, Gooren LJ. Lower urinary-tract symptoms and testosterone in elderly men. World J Urol. 2008; 26:359–364.
Article21. Rohrmann S, Nelson WG, Rifai N, Kanarek N, Basaria S, Tsilidis KK, et al. Serum sex steroid hormones and lower urinary tract symptoms in Third National Health and Nutrition Examination Survey (NHANES III). Urology. 2007; 69:708–713.
Article22. Takyu S. Effects of testosterone on the autonomic receptor-mediated function in lower urinary tract from male rabbits. Nihon Hinyokika Gakkai Zasshi. 1993; 84:330–338.
Article23. Christ GJ, Hodges S. Molecular mechanisms of detrusor and corporal myocyte contraction: identifying targets for pharmacotherapy of bladder and erectile dysfunction. Br J Pharmacol. 2006; 147:Suppl 2. S41–S55.
Article24. Rees RW, Foxwell NA, Ralph DJ, Kell PD, Moncada S, Cellek S. Y-27632, a Rho-kinase inhibitor, inhibits proliferation and adrenergic contraction of prostatic smooth muscle cells. J Urol. 2003; 170:2517–2522.
Article25. Ehrén I, Adolfsson J, Wiklund NP. Nitric oxide synthase activity in the human urogenital tract. Urol Res. 1994; 22:287–290.26. Yassin DJ, El Douaihy Y, Yassin AA, Kashanian J, Shabsigh R, Hammerer PG. Lower urinary tract symptoms improve with testosterone replacement therapy in men with late-onset hypogonadism: 5-year prospective, observational and longitudinal registry study. World J Urol. 2014; 32:1049–1054.
Article27. Holmäng S, Mårin P, Lindstedt G, Hedelin H. Effect of long-term oral testosterone undecanoate treatment on prostate volume and serum prostate-specific antigen concentration in eugonadal middle-aged men. Prostate. 1993; 23:99–106.
Article28. Gooren LJ. A ten-year safety study of the oral androgen testosterone undecanoate. J Androl. 1994; 15:212–215.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Korean Herbal Formula (Modified Ojayeonjonghwan) on Androgen Receptor Expression in an Aging Rat Model of Late Onset Hypogonadism
- Effects of Vitamin A and Bacillus Calmette -Guerin (BCG) Combination on Experimental Bladder Cancer
- Immunohistochemical Assay of Androgen Receptor in Prostate Cancer
- The Effects of Androgen on Androgen Receptor, Apoptosis and Proliferation in the Penile Erectile Tissue of Adult Rat
- Relationship of Androgen Receptor and p53 Protein Expession to HormonalTherapy in Advanced Prostatic Cancer