Korean J Urol.
2007 Apr;48(4):444-451. 10.4111/kju.2007.48.4.444.
The Histological Changes and Expression of Hypoxia Inducible Factor-1alpha and Vascular Endothelial Growth Factor according to the Differential Renal Function during Total Ureteral Obstruction in the Rabbit Model
- Affiliations
-
- 1Department of Urology, Veterans Hospital, Busan, Korea.
- 2Department of Urology, College of Medicine, Pusan National University, Busan, Korea. lsd@pusan.ac.kr
- 3Department of Nuclear Medicine, College of Medicine, Pusan National University, Busan, Korea.
- 4Department of Pathology, College of Medicine, Pusan National University, Busan, Korea.
- 5Department of Medical Research Institute, College of Medicine, Pusan National University, Busan, Korea.
- KMID: 1997114
- DOI: http://doi.org/10.4111/kju.2007.48.4.444
Abstract
- PURPOSE
The renal histological and hemodynamic changes and the expressions of hypoxia inducible factor-1alpha (HIF-1alpha) and vascular endothelial growth factor (VEGF) were evaluated according to the differential renal function (DRF) during total ureteral obstruction (TUO) in a rabbit model.
MATERIALS AND METHODS
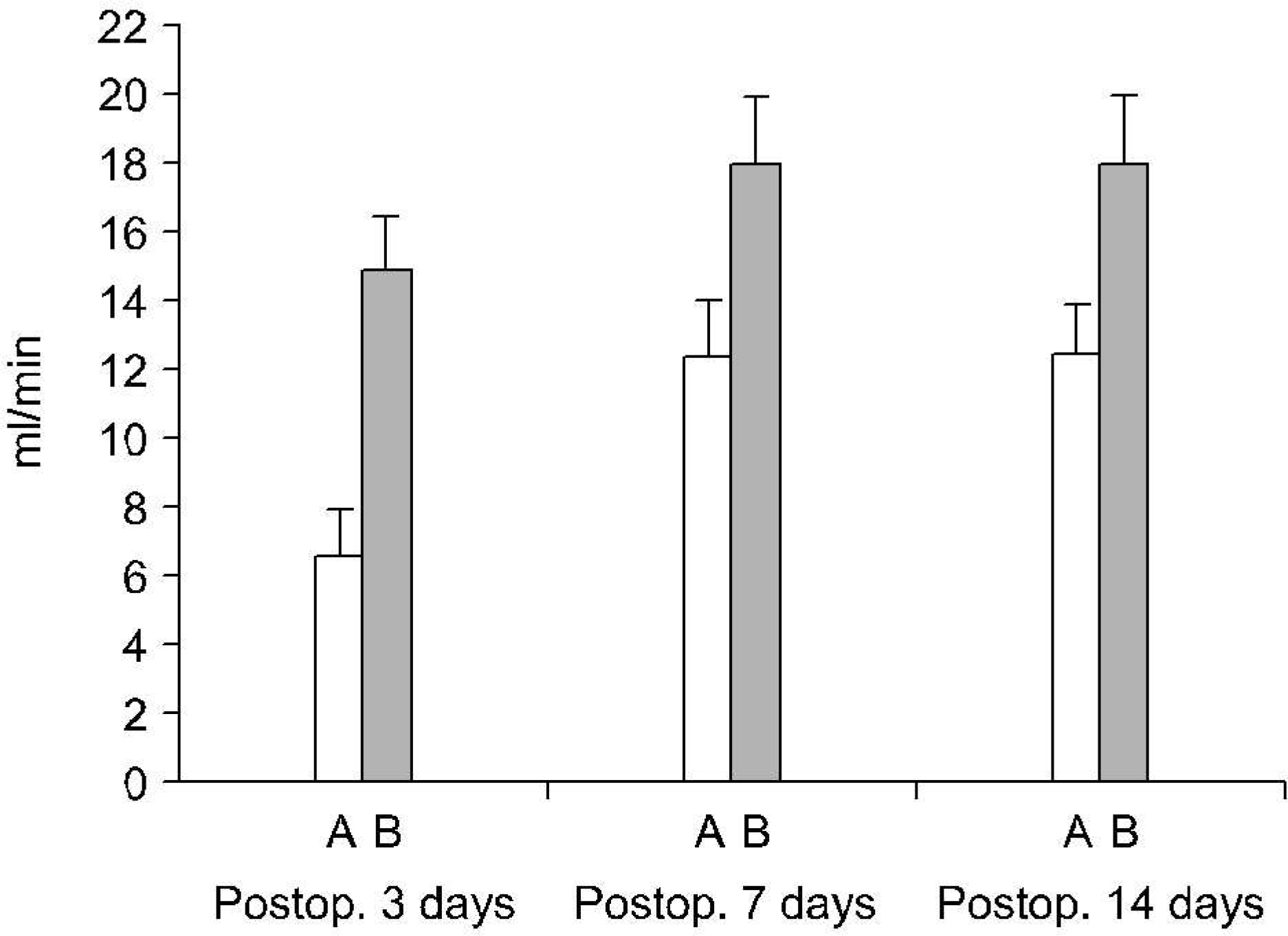
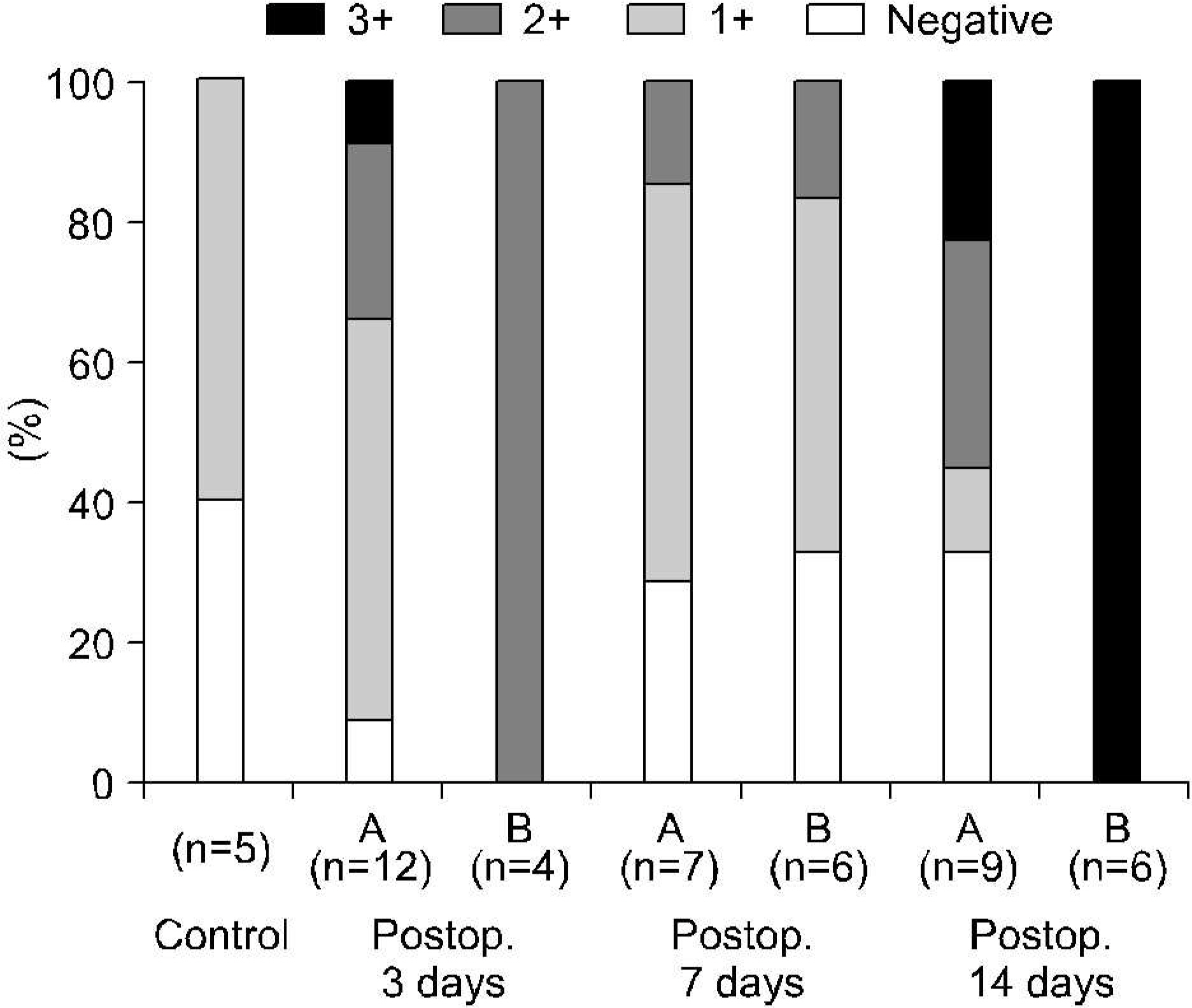
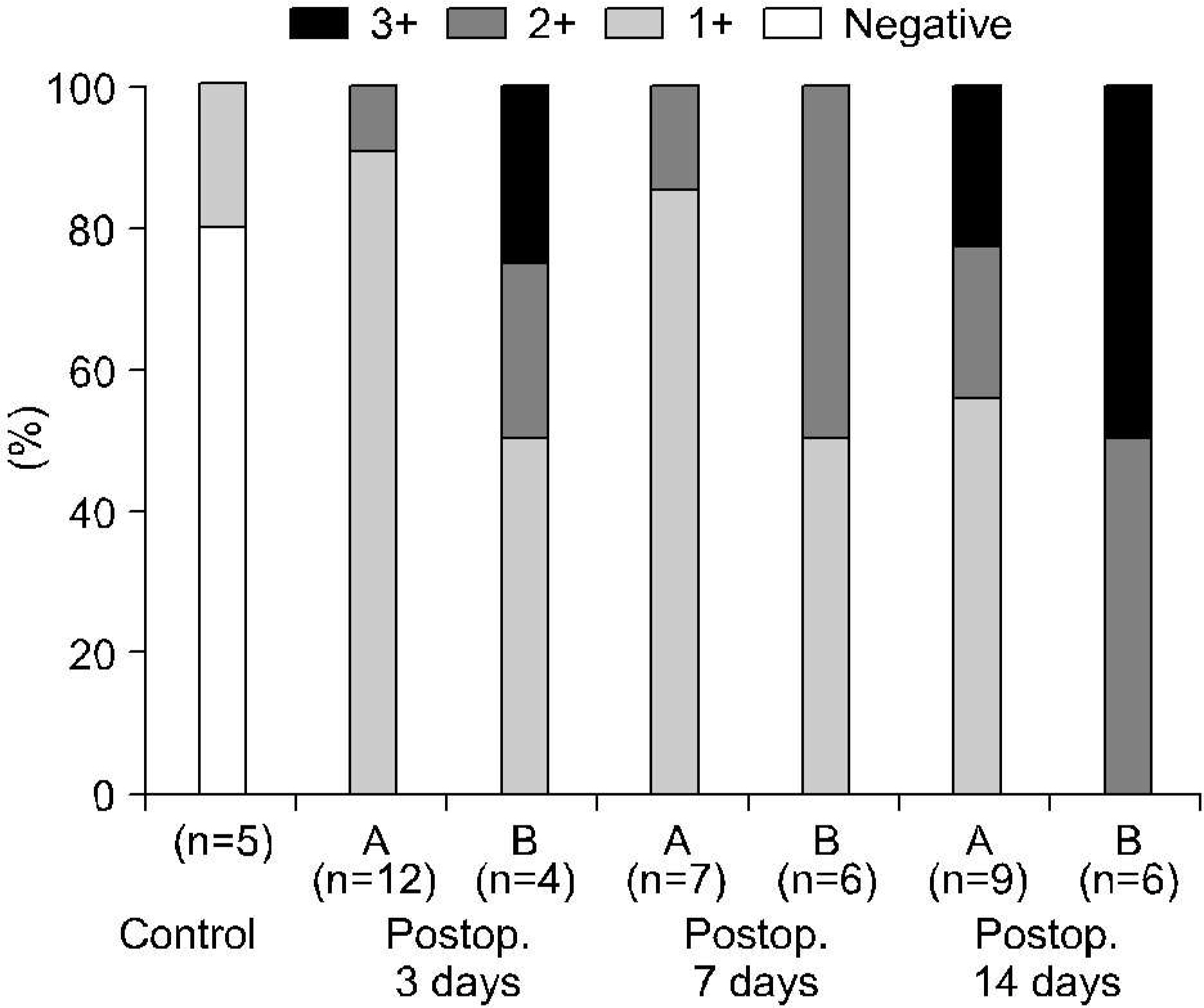
In forty-nine control (5) and 16 experimental rabbits (16 in TUO 3 days, 13 in TUO 7 days and 15 in TUO 14 days), the renal blood flow (RBF) and 99mTc-DTPA renal scan were measured both before and after TUO. The cut-off of the DRF group was 40%. The histological changes and expressions of HIF-1alpha and VEGF were evaluated using H&E and immunohistochemical stain, respectively.
RESULTS
The entire control group demonstrated more than 40% DRF. Contrary to the control group, the DRF was less than 40% in 4 (25%), 7 (53%) and 6 rabbits (40%) in TUO 3, 7 and 14 day groups, respectively. The postobstructive compared to preobstructive RBF was decreased in each group. The RBF was more decreased in the lower than the higher DRF group (more than 40%) in all of the experimental groups. Abnormal histological changes were more prominent in the experimental groups, and increased with the obstruction time. However, there was no difference in relation to the DRF. The expressions of HIF-1alpha and VEGF were more prominent in the experimental and lower DRF groups.
CONCLUSIONS
During acute TUO, the decreased RBF and hypoxia may play a role in preservation of the DRF.
Keyword
MeSH Terms
Figure
Reference
-
1.Fitzgerald J., Chou SY., Wahid A., Porush JG. Regional expression of inducible nitric oxide syndiase in the kidney in dogs with unilateral ureteral obstruction. J Urol. 2001. 166:1524–9.2.Lanzone JA., Gulmi FA., Chou SY., Mooppan UM., Kim H. Renal hemodynamics in acute unilateral ureteral obstruction: contribution of endothelium-derived relaxing factor. J Urol. 1995. 153:2055–9.
Article3.Wenger RH., Gassmann M. HIF-1 and die molecular response to hypoxia in mammals. Storey KB, editor. editor.Environmental stress and gene regulation. 1st ed.Oxford: BIOS Scientific Publishers;1999. p. 25–45.4.Semenza GL. Regulation of mammalian 02 homeostasis by hypoxia inducible factor 1. Annu Rev Cell Dev Biol. 1999. 15:551–78.5.Feldser D., Agani F., Iyer NV., Pak B., Ferreira G., Semenza GL. Reciprocal positive regulation of hypoxia inducible factor 1 a and insulin like growth factor 2. Cancer Res. 1999. 59:3915–8.6.Lear S., Silva P., Kelley VE., Epstein FH. Prostaglandin E2 inhibits oxygen consumption in rabbit medullary thick ascending limb. Am J Physiol. 1990. 258:1372–8.
Article7.Heyman SN., Brezis M., Epstein FH., Spokes K., Silva P., Rosen S. Early renal medullary hypoxic injury from radiocontrast and indomethacin. Kidney Int. 1991. 40:632–42.
Article8.Cadnapaphomchai P., Aisenbrey G., McDonald KM., Burke TJ., Schrier RW. Prostaglandin-mediated hyperemia and renin-mediated hypertension during acute ureteral obstruction. Prostaglandins. 1978. 16:965–71.
Article9.Kim KM., Bogaert GA., Nguyen HT., Borirakchanyavat S., Kogan BA. Hemodynamic changes after complete unilateral ureteral obstruction in the young lamb. J Urol. 1997. 158:1090–3.
Article10.Yarger WE., Schocken DD., Harris RH. Obstructive nephropathy in the rat: possible roles for the renin-angiotensin system, prostaglandins, and thromboxanes in postobstructive renal function. J Clin Invest. 1980. 65:400–12.
Article11.Wahlberg J., Karlberg L., Persson AE. Total and regional renal blood flow during complete unilateral ureteral obstruction. Acta Physiol Scand. 1984. 121:111–8.
Article12.Chun YS., Kim MS., Park JW. Oxygen-dependent and -independent regulation of HIF-1 a. J Korean Med Sci. 2002. 17:581–8.13.Ferrara N., Henzel JW. Pituitary follicular cells secreate a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989. 161:851–8.14.Jinno K., Tanimizu M., Hyodo I., Nishikawa Y., Hosokawa Y., Doi T, et al. Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma. J Gastroenterol. 1998. 33:376–82.15.Yoshiji H., Kuriyama S., Ways DK., Yoshii J., Miyamoto Y., Kuwata M, et al. Protein kinase C lies on the signaling pathway for vascular endothelial growth factor-mediated tumor development and angiogenesis. Cancer Res. 1999. 59:4413–8.16.Zhong H., De Marzo AM., Laughner E., Lim M., Hilton DA., Zagzag D, et al. Overexpression of hypoxia-inducible factor 1 沙in common human cancers and their metastasis. Cancer Res. 1999. 59:5830–5.17.Turner KJ., Moore JW., Jones A., Taylor CF., Cuthbert-Heavens D., Han C, et al. Expression of hypoxia-inducible factors in human renal cancer: relationship to angiogenesis and to the von Hippel-Lindau gene mutation. Cancer Res. 2002. 62:2957–61.18.Josephson S., Robertson B., Claesson G., Wikstad I. Experimental obstructive hydronephrosis in newborn rats. I. Surgical technique and long term morphologic effects. Invest Urol. 1980. 17:478–82.19.Claesson G., Josephson S., Robertson B. Experimental partial ureteric obstruction in newborn rats. IV. Do the morphological effects progress continuously? J Urol. 1983. 130:1217–22.20.Zelman SJ., Zenser TV., Davis BB. Renal growth in response to unilateral ureteral obstruction. Kidney Int. 1983. 23:594–8.
Article21.Steinhardt GF., Ramon G., Salinas-Madrigal L. Glomerulosclerosis in obstructive uropathy. J Urol. 1988. 140:1316–8.
Article22.Perlmutter AD., Kroovand RL., Lai YW. Management of ureteropelvic obstruction in the first year of life. J Urol. 1980. 123:535–7.
Article23.Kruerger RP., Ash JM., Silver MM., Kass EJ., Gilmour RF., Alton DJ, et al. Primary hydronephrosis. Assessment of diuretic renography, pelvis perfussion pressure, operative findings, and renal and ureteral histology. Urol Clin North Am. 1980. 7:231–42.24.Stock JA., Krous HF., Hefferman J., Parker M., Kaplan GW. Correlation of renal biopsy and radionuclide renal scan differentiational function in patients with unilateral ureteropelvic junction obstruction. J Urol. 1995. 154:716–8.25.Elder JS., Stansbrey R., Dahms BB., Selzman AA. Renal histological changes secondary to ureteropelvic junction obstruction. J Urol. 1995. 154:719–22.
Article26.Pascual L., Oliva J., Vega-P J., Prindpi I., Valles P. Renal histology in ureteropelvic junction obstruction: are histological changes a consequence of hyperfiltration? J Urol. 1998. 160:976–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Relationship between Expression of Hypoxia Inducible Factor-1alpha or Vascular Endothelial Growth Factor and Histopathological Characteristics in Human Renal Cell Carcinoma
- HIF-1alpha, iNOS and VEGF Expression of Contralateral Kidney during Acute Stage in Complete Ureteral Obstruction of Rat Model
- The Relationship between Expression of Hypoxia Inducible Factor-1alpha or Vascular Endothelial Growth Factor and Histopathological Characteristics in Human Transitional Bladder Cancer
- Expression of Hypoxia-Inducible Factor-1alpha(HIF-1alpha) in the Experimental Model of Choroidal Neovascularization
- Penile Expression of Hypoxia-inducible Factor-1alpha, Vascular Endothelial Growth Factor, and Nitric Oxide Synthase in the Type II Diabetic Rat