J Korean Acad Conserv Dent.
2006 Jul;31(4):290-299. 10.5395/JKACD.2006.31.4.290.
The effect of ethylene glycol analogs on mechanical properties of moist demineralized dentin matrix
- Affiliations
-
- 1Department of Conservative Dentistry, School of Dentistry, Chonbuk National University, Korea. lkw@chonbuk.ac.kr
- 2Department of Conservative Dentistry, College of Dentistry, Chosun University, Korea.
- KMID: 1986872
- DOI: http://doi.org/10.5395/JKACD.2006.31.4.290
Abstract
OBJECTIVES
The purpose of this study is to evaluate the effect of ethylene glycol analogs on modulus of elasticity and ultimate tensile strength of moist, demineralized dentin matrix.
METHODS
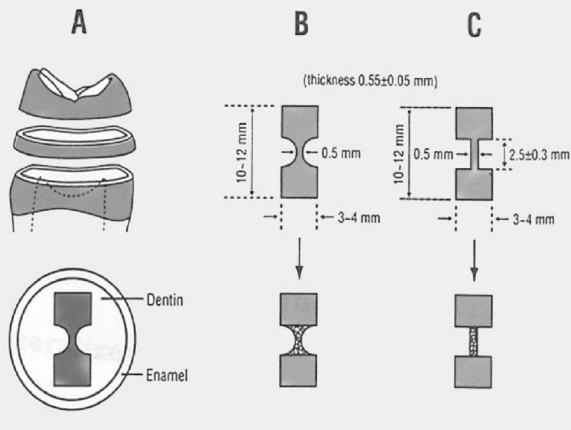
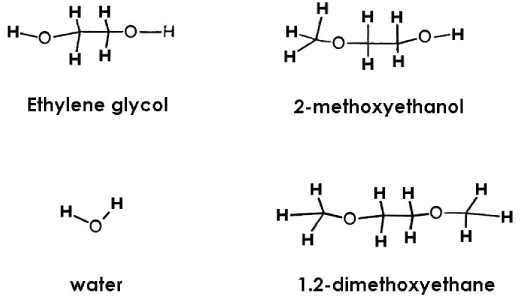
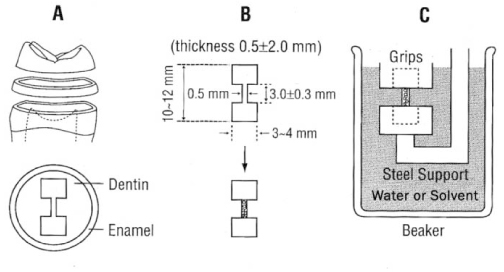
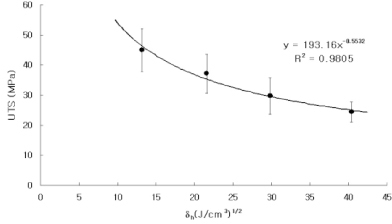
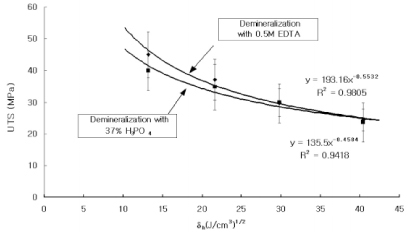
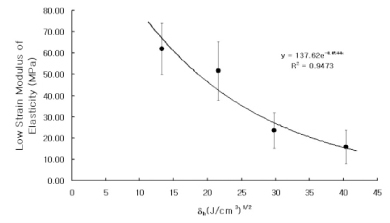
Dentin disks 0.5 mm thick were prepared from mid-coronal dentin of extracted, unerupted, human third molars. "I" beam and hour-glass shaped specimens were prepared from the disks, the ends protected with nail varnish and the central regions completely demineralized in 0.5M EDTA for 5 days. Ultimate tensile stress (UTS) and low strain modulus of elasticity (E) were determined with specimens immersed for 60 min in distilled water (H2O), ethylene glycol (HO-CH2-CH2-OH), 2-methoxyethanol (H3CO-CH2-CH2-OH), and 1,2-dimethoxyethane (H3CO-CH2-CH3-OCH3) prior to testing in those same media. Modulus of elasticity was measured on the same specimens in a repeated measures experimental design. The results were analyzed with a one-way ANOVA on ranks, followed by Dunn's test at alpha = 0.05. Regression analysis examined the relationship between UTS or E and hoy's solubility parameter for hydrogen bonding (delta(h)) of each solvent.
RESULTS
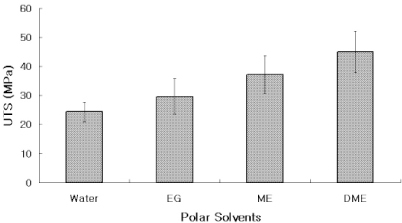
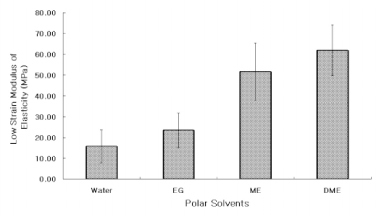
The UTS of demineralized dentin in water, ethylene glycol, 2-methoxyethanol, and 1,2-dimethoxyethane was 24 (3), 30 (5), 37 (6), and 45 (6) MPa, x (SD) N = 10. Low strain E for the same media were 16 (13), 23 (14), 52 (24), and 62 (22) MPa. Regression analysis of UTS vs delta(h) revealed a significant (p < 0.0001, r = -0.99, R2 = 0.98) inverse, exponential relationship. A similar inverse relationship was obtained between low strain E vs delta(h) (p < 0.0005, r = -0.93, R2 = 0.86). SIGNIFICANCE: The tensile properties of demineralized dentin are dependent upon the hydrogen bonding ability of polar solvents (delta(h)). Solvents with low delta(h) values may permit new interpeptide H-bonding in collagen that increases its tensile properties. Solvents with high delta(h) values prevent the development of these new interpeptide H-bonds.
MeSH Terms
Figure
Reference
-
1. Nakabayashi N, Pashley DH. Hybridization of Dental Hard Tissues. 1998. 1st edit. Tokyo: Quintessence Publishers;42–56.2. Maciel KT, Carvalho RM, Ringle RD, Preston CD, Russell CM, Pashley DH. The effects of acetone, ethanol, HEMA and air on the stiffness of human decalcified dentin matrix. J Dent Res. 1996. 75:1851–1858.
Article3. Pashley DH, Agee KA, Nakajima M, Tay FR, Carvalho RM, Terada Rss, Harmon FJ, Lee KW, Rueggeberg RA. Solvent-induced dimensional changes in EDTA demineralized dentin matrix. J Biomed Mater Res. 2001. 56(2):273–281.4. Barton Allan FM. Chapter 5: Expanded cohesion parameters. CRC Handbook of Solubility Parameters and Other Cohesion Parameters. 1991. 2nd ed. Boca Raton: CRC Press;98–103. 250–257.5. Pashley DH, Agee KA, Carvalho RM, Lee KW, Tay FR, Callison TE. Effects of water and water-free polar solvents on the tensile properties of demineralized dentin. Dent Mater. 2003. 19(5):347–352.
Article6. Asmussen E, Uno S. Solubility parameters, fractional polarities, and bond strengths of some intermediary resins used in dentin bonding. J Dent Res. 1993. 72(3):558–565.
Article7. Barton Allan FM. Handbook of Solubility Parameters and other Cohesion Parameters. 1991. 2nd ed. CRC;123–137.8. Hoy KL. Tables of Solubility Parameters, Solvent and Coatings Materials Research and Development Department. 1985. Union Carbide Co..9. Pashley DH, Zhang Y, Agee KA, Rouse CJ, Carvalho RM, Russell CM. Permeability of demineralized dentin to HEMA. Dent Mater. 2000. 16:7–14.
Article10. Asmussen E, Hansen EK, Pentzfeldt A. Influence of the solubility parameter of intermediary resin on the effectiveness of the Gluma bonding system. J Dent Res. 1991. 70(9):1290–1293.
Article11. Miller RG, Bowles CQ, Chappelow CC, Eick JD. Application of solubility parameter theory to dentin-bonding and adhesive strength correlations. J Biomed Mater Res. 1998. 41:237–243.
Article12. Sasaki N, Odajima S. Stress-strain curve and Young's modulus of a collagen molecules as determined by the X-ray diffraction technique. J Biomech. 1996. 29:655–658.
Article13. Craig R. Restorative Dental materials. 8th ed. 1989. Mosby;65–112.14. Sano H, Ciucchi B, Takatsu T, Russell CM, Pashley DH. Tensile properties of mineralized and demineralized human and bovine dentin. J Dent Res. 1994. 73(6):1205–1211.
Article15. Perdigão J, Lopes M, Gerableri S, Lopes GC, Garcia-Godoy F. Effect of a sodium hypochlorite gel on dentin bonding. Dent Mater. 2000. 16:311–323.
Article16. Zhang Y, Agee K, Nor J, Carvalho R, Sachar B, Russell C, Pashley D. Effects of acid-etching on the tensile properties of demineralized dentin matrix. Dent mater. 1998. 14:222–228.
Article17. Pashley DH, Carvalho RM, Tay FR, Agee KA, Lee KW. Solvation of dried dentin matrix by water and other polar solvents. Am J Dent. 2002. 15(2):97–102.18. Knott L, Bailey AJ. A review of their chemistry, function and clinical relevance. Bone. 1998. 22:181–187.
Article19. Silver FH, Christiansen D, Snowhill PB, Chen Y, Landis WJ. The role of mineral in the storage of elastic energy in turkey tendons. Biomacromolecules. 2000. 1:180–185.
Article20. Tay FR, Carvalho RM, Yiu CKY, King NM, Zhang Y, Agee K, Bouillaguet S, Pashley DH. Mechanical disruption of dentin collagen fibrils during resin-dentin bond testing. J Adhes Dent. 2000. 2:175–192.21. Kato YP, Christiansen DL, Hahn RA, Shieh SJ, Goldstein JD, Silver FH. Mechanical properties of collagen fibers: A comparison of reconstituted and rat tail tendon fibers. Biomaterials. 1989. 10:38–42.
Article22. Finger WJ, Inoue M, Asmussen E. Effects of wetability of adhesive resins on bonding to dentin. Am J Dent. 1994. 7:35–38.23. Chappelow CC, Power MD, Bowles CQ, Miller RG, Pinzino CS, Eick JD. Novel priming and cross-linking systems for use with isocyano-methacrylate dental adhesives. Dent Mater. 2000. 16:396–405.
Article24. Kato G, Nakabayashi N. The durability of adhesion to phosphoric acid etched, wet dentin substrates. Dent Mater. 1998. 14(5):347–352.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone Induction by Demineralized Dentin Matrix in Nude Mouse Muscles
- Acute renal failure and delayed neuropathy due to anti-freeze ingestion
- Demineralized Dentin Matrix for Dental and Alveolar Bone Tissues Regeneration: An Innovative Scope Review
- Study of bone healing pattern in extraction socket after application of demineralized dentin matrix material
- Recovery of Acute Renal Failure Secondaruy to Ethylene Glycol Intoxicity