J Korean Assoc Oral Maxillofac Surg.
2011 Oct;37(5):365-374.
Study of bone healing pattern in extraction socket after application of demineralized dentin matrix material
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, College of Dentistry, Dankook University, Choenan, Korea. lee201@dankook.ac.kr
Abstract
- INTRODUCTION
Research on dental bone graft material has been actively conducted. Recently, demineralized dentin matrix material has been developed and introduced. This study examined the effect of demineralized dentin matrix material on bone healing.
SUBJECTS AND METHODS
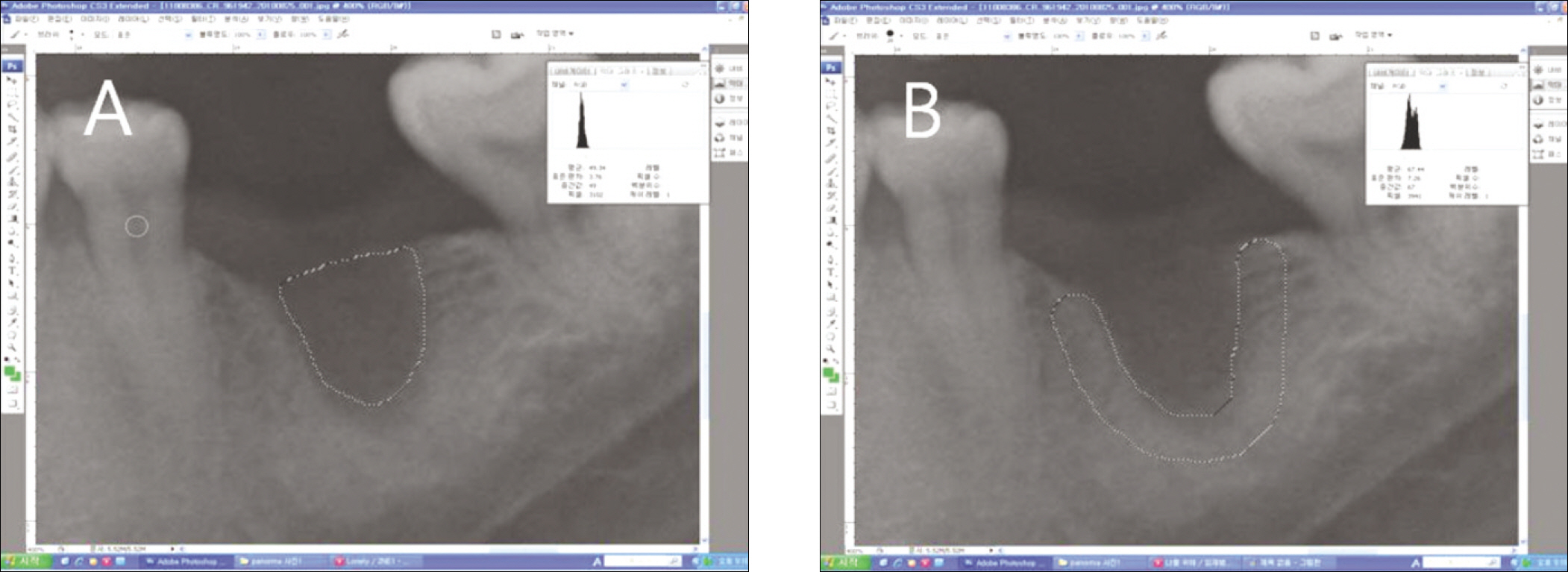
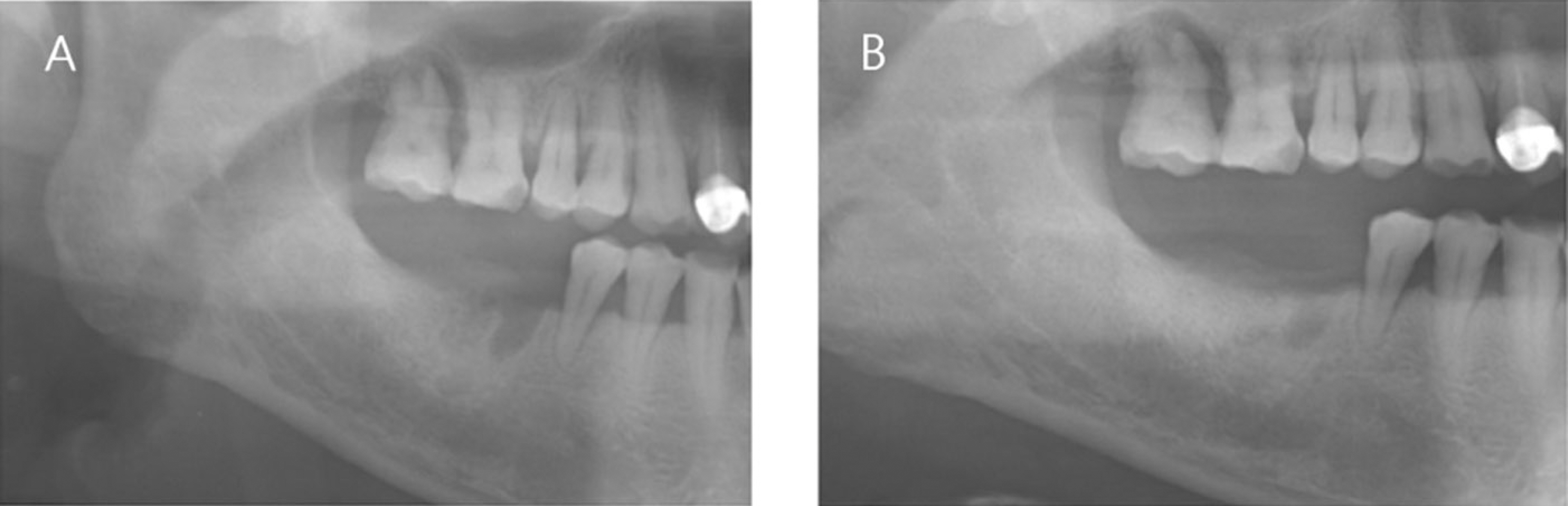
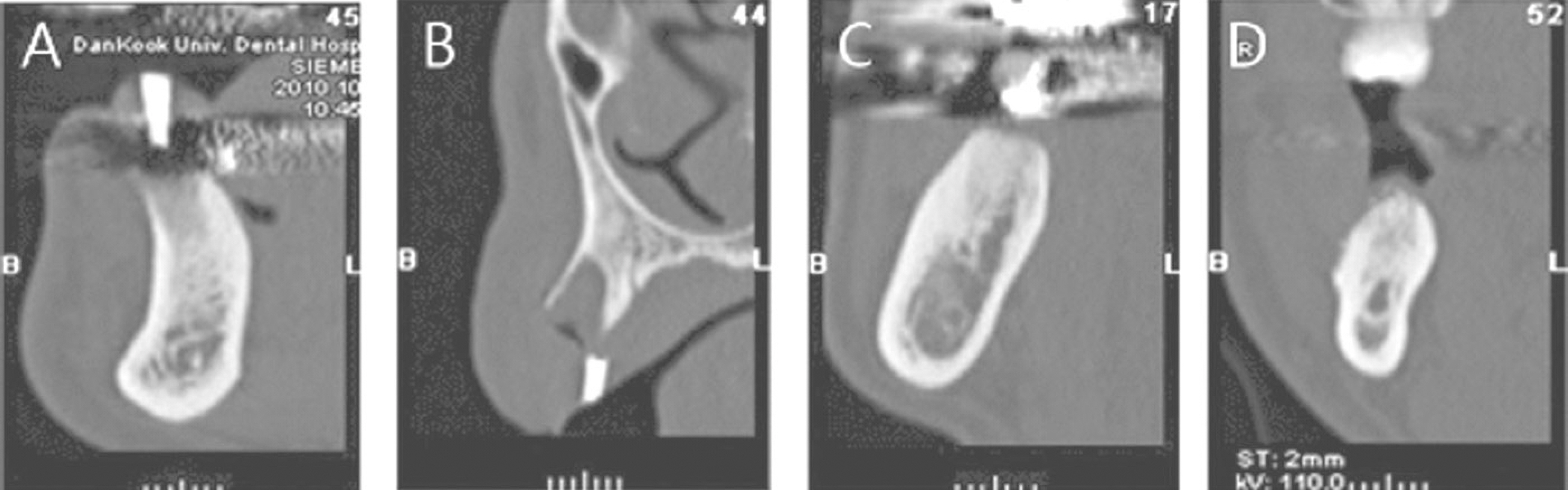
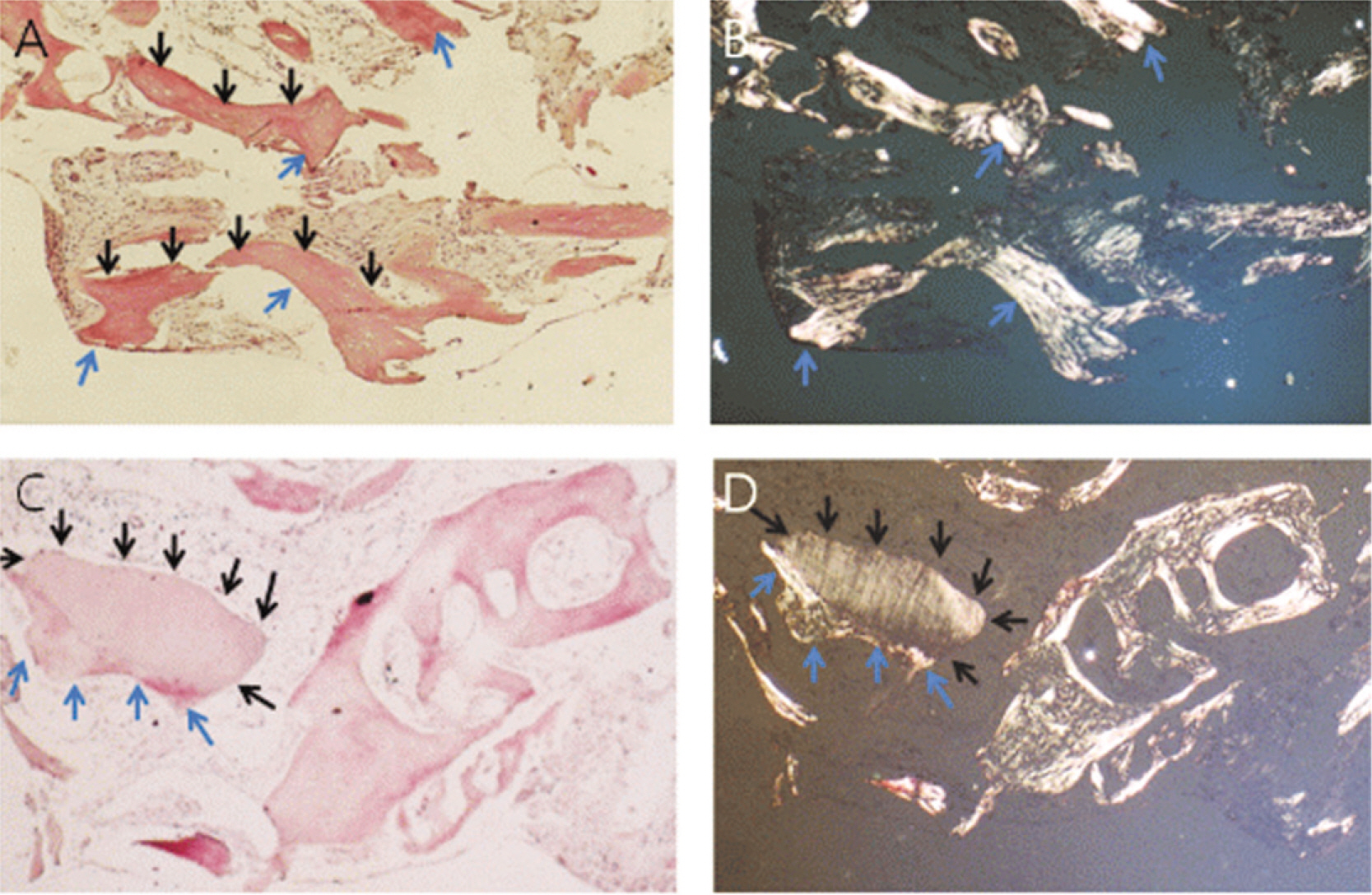
The patients who received no treatment after extraction were used as the control group and patients who underwent demineralized dentin matrix material application in the extraction socket after extraction were used as the experimental group. Panorama radiography was performed at the baseline and at 3.5 months after graft material placement and CT was taken at 3.5 months after graft material placement for a radiologic evaluation. Bony tissue specimens were collected from the alveolar crest in the middle of the extraction socket using a 2 mm trephine bur after 3.5 months for the histology and hostomorphometric study.
RESULTS
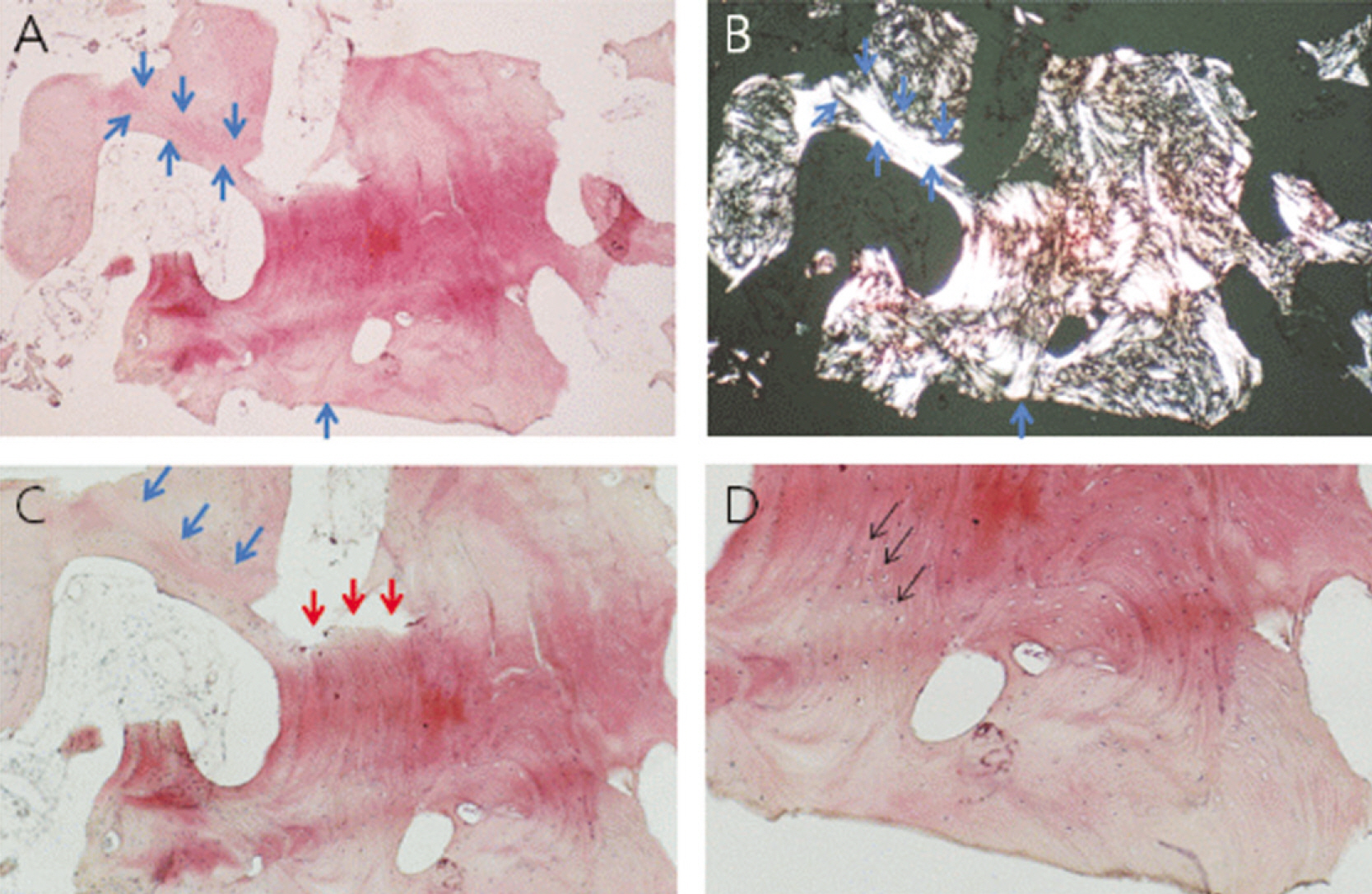
1. On the panoramic view, a higher bone density was observed in the subject group. 2. On the panoramic view, the bone density increased significantly in the extraction socket, from the baseline to 3.5 months: a 7 and 10 gray-level scale was observed in the control and experimental group, respectively (P<0.05). 3. The CT view evaluation at 3.5 months revealed significantly higher bone density in the subject group than the control group (P<0.05). 4. The histological findings showed more active new bone and lamellar bone formation in the subject group. Dentin with osteoinduction ability and enamel with osteoconduction ability appeared. 5. On histomorphometric analysis, the subject group showed significantly more new bone, lamellar bone area and lower soft tissue area (P<0.05). The difference between the groups was significant (P<0.05).
CONCLUSION
Bone healing was improved after the application of demineralized dentin matrix material and there was active new bone and lamellar bone formation.
MeSH Terms
Figure
Reference
-
References
1. Myoung MR, Kim MR, Kim SJ. A retroapective study of the surgical success and vertical bone resorption rate after autogenous block onlay graft in posterior maxilla. J. Kor. Oral Maxillofac. Surg. 2009; 35:340–5.2. Quattlebaum JB, Mellonig JT, Hensel NF. Antigenicity of freeze-dried cortical bone allograft in human periodontal osseous defects. J Periodontol. 1988; 59:394–7.3. Schwartz Z, Mellonig JT, Carnes DL Jr, de la Fontaine J, Cochran DL, Dean DD, et al. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation. J Periodontol. 1996; 67:918–26.
Article4. Sogal A, Tofe AJ. Risk assessment of bovine spongiform encephalopathy transmission through bone graft material derived from bovine bone used for dental applications. J Periodontol. 1999; 70:1053–63.
Article5. Han T, Carranza FA Jr, Kenney EB. Calcium phosphate ceramics in dentistry: a review of the literature. J West Soc Periodontol Periodontal Abstr. 1984; 32:88–108.6. Block MS, Degen M. Horizontal ridge augmentation using human mineralized particulate bone: preliminary results. J Oral Maxillofac Surg. 2004; 62(9 supple 2):67–72.
Article7. Kim YK, Kim SG, Lim SC. The comparative study of guided bone regeneration using various of bone graft materials. J Kor Oral Maxillofac Surg. 2007; 33:350–8.8. Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:496–503.
Article9. Min BM. Oral biochemistry. 1st ed.Seoul: Daehan Narae Publishing;2007.10. Bhaskar SN. Orban's oral histology and embryology. 9th ed.St. Louis: Mosby Co.;1980.11. Yeomans JD, Urist MR. Bone induction by decalcified dentin implanted into oral, osseous, and muscle tissues. Archiv Oral Biol. 1967; 12:999–1008.12. Kim SG, Yeo HH, Kim YK. Grafting of large defects of the jaws with a particulate dentin-laster of Paris combination. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 88:22–5.13. Kim SG, Chung CH, Kim YK. Grafting defects using a particulate dentin-plaster of Paris combination for implant placement: a case report. Hosp Dent(Tokyo). 2001; 13:127–30.14. Kim SG, Chung CH, Kim YK, Park JC, Lim SC. The use of particulate dentin-plaster of Paris combination with/without platelet-rich plasma in the treatment of bone defects around implants. Int J Oral Maxillofac Implants. 2002; 17:86–94.15. Park SS, Kim SG, Lim SC, Ong JL. Osteogenic activity of the mixture of chitosan and particulate dentin. J Biomed Materials Res A. 2008; 87:618–23.
Article16. Kim SG, Kim HK, Lim SC. Combined implantation of particulate dentin, plaster of Paris, and a bone xenograft(Bio-Oss) for bone regeneration in rats. J Craniomaxillofac Surg. 2001; 29:282–8.17. Kim YK, Yun PY, Kim SG, Lim SC. Sinus bone graft using combination of autogenous bone and Bio-Oss comparison of healing according to the ratio of autogenous bone. J Korean Oral Maxillofac Surg. 2007; 33:654–9.18. Maiorana C, Beretta M, Salina S, Santoro F. Reduction of autogenous bone graft resorption by means of bio-oss coverage: a prospective study. Int J Periodontics Restorative Dent. 2005; 25:19–25.19. Arau′jo MG, Sonohara M, Hayaclbara R, Cardaropoll G, Lindhe J. Lateral ridge augmentation by the use of grafts comprised of autologous bone or a biomaterial. An experiment in the dog. J Clin Periodontol. 2002; 29:1122–31.20. Camelo M, Nevins ML, Lynch SE, Schenk RK, Simion M, Nevins M. Periodontal regeneration with an autogenous bone Bio-Oss composite graft and a Bio-Guide Membrane. Int J Periodontics Restorative Dent. 2001. 109–19.21. Pinholt EM, Haanaes HR, Donath K, Bang G. Titanium implant insertion into dog alveolar ridges augmented by allogenic material. Clin Oral Implants Res. 1994; 5:213–9.
Article22. Jensen OT, Greer RO Jr, Johnson L, Kassebaun D. Vertical guided bone graft augmentation in a new canine mandible model. Int J Oral Maillofac Implants. 1995; 10:335–44.23. Gomes MF, dos Anjos MJ, Nogueira TO, Guimarães SA. Histologic evaluation of the osteoinduction property of autogenous demineralized dentin matrix on surgical bone defects in rabbit skulls using human amniotic membrane for guided bone regeneration. Int J Oral Maxillofac Implants. 2001; 16:563–71.24. Catanzaro-Guimarães SA, Catanzaro Guimarães BP, Garcia RB, Alle N. Osteogenic potential of autogenic demineralized dentin implanted in bony defects in dog. Int J Oral Maxillofac Surg. 1986; 15:160–9.25. Ike M, Urist MR. Recycled dentin root matrix for a carrier of recombinant human bone morphogenetic protein. J Oral Implantol. 1998; 24:124–32.
Article26. Gao J, Symons AL, Bartold PM. Expression of transforming growth factor-beta 1 (TGF-beta1) in the developing periodontium of rats. J Dent Res. 1998; 77:1708–16.27. Saygin NE, Tokiyasu Y, Giannobile WV, Somerman MJ. Growth factors regulate expression of mineral associated genes in cementoblasts. J Periodontol. 2000; 71:1591–600.
Article28. Gomes MF, dos Anjos MJ, Nogueira Tde O, Catanzaro Guimarães SA. Autogenous demineralized dentin matrix for tissue engineering applications: radiographic and histomorphometric studies. Int J Oral Maxillofac Implants. 2002; 17:488–97.29. Carvalho VA, Tosello Dde O, Salgado MA, Gomes MF. Histomorphometric analysis of hemogenous demineralized dentin matrix as oteopromotive material in rabbit mandibles. Int J Oral Maxillofac Implants. 2004; 19:679–86.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone Induction by Demineralized Dentin Matrix in Nude Mouse Muscles
- Demineralized Dentin Matrix for Dental and Alveolar Bone Tissues Regeneration: An Innovative Scope Review
- Various autogenous fresh demineralized tooth forms for alveolar socket preservation in anterior tooth extraction sites: a series of 4 cases
- Collagen biology for bone regenerative surgery
- EFFECT OF ELECTRICAL STIMULATION ON BONE FORMATION IN THE EXTRACTION SOCKET OF RAT