Int J Stem Cells.
2014 Nov;7(2):143-152. 10.15283/ijsc.2014.7.2.143.
Growth Properties and Pluripotency Marker Expression of Spontaneously Formed Three-dimensional Aggregates of Human Adipose-derived Stem Cells
- Affiliations
-
- 1Latvian Biomedical Research and Study Centre, Riga, Latvia. ance.bogdanova@biomed.lu.lv
- KMID: 1974602
- DOI: http://doi.org/10.15283/ijsc.2014.7.2.143
Abstract
- BACKGROUND AND OBJECTIVES
Recent findings suggest that therapeutic potential of mesenchymal stem cells (MSCs) could be increased through aggregation into three-dimensional (3D) bodies, and different culture methods have been employed to obtain 3D spheroids of MSCs. In the current study we report accidentally encountered spontaneous formation of adipose-derived stem cell (ASC) bodies in standard ASC culture of a single donor.
METHODS AND RESULTS
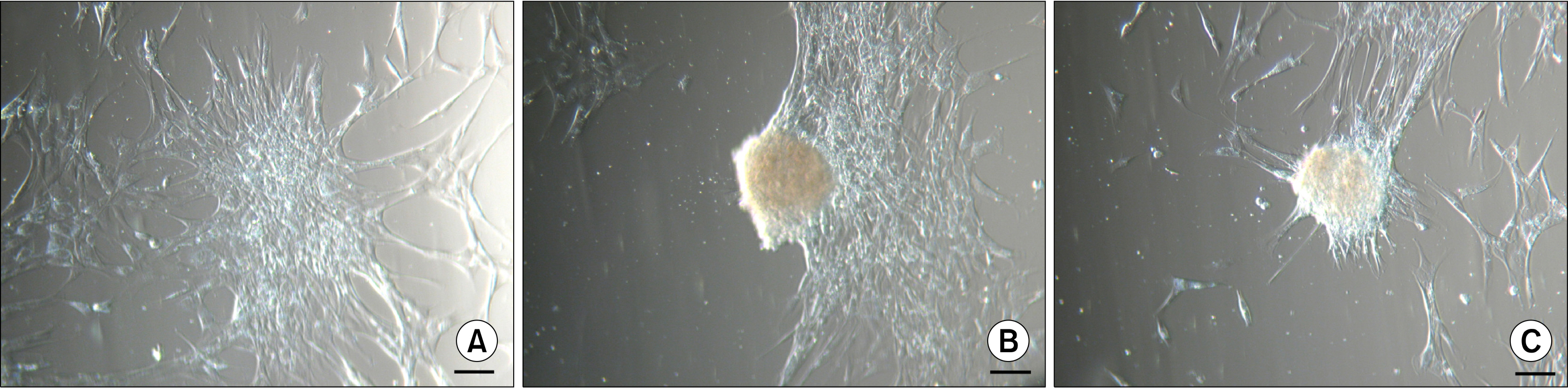
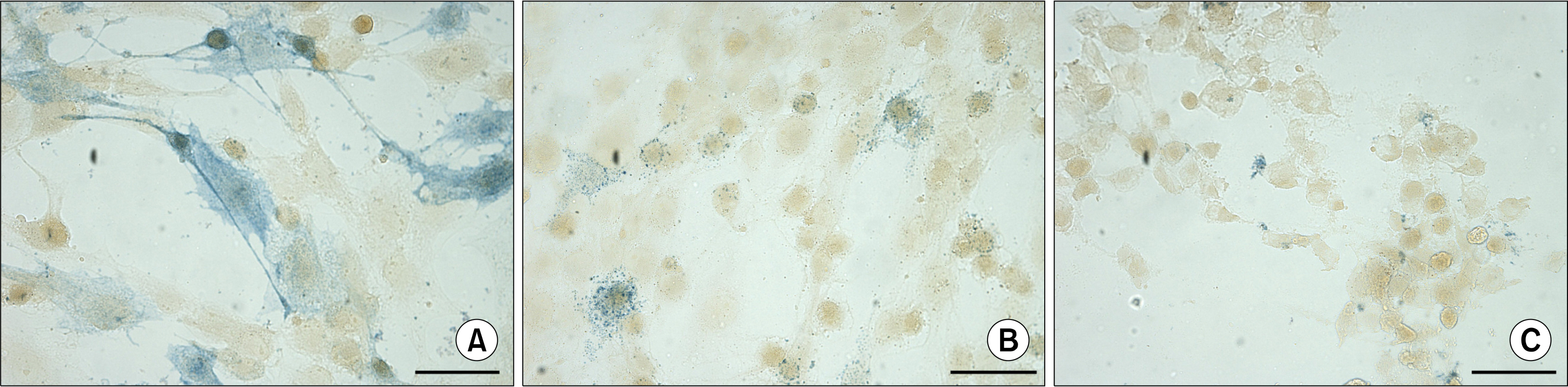
Human ASCs from passages 1 to 3, cultured in a medium containing 5% autologous serum (AS), spontaneously clustered and formed floating 3D bodies. After a transfer of floating ASC bodies onto new adherent plastic dish, they attached to the surface and gradual migration of spindle-shaped ASCs out of the bodies was detected. A substitution of AS with allogeneic sera did not hinder this ability, but commercial medium containing fetal bovine serum delayed the process. Substantial part of ASCs surrounding transferred ASC bodies showed alkaline phosphatase (AP) activity, while ASC aggregates were AP negative. Similar 3D bodies formed when ASCs were grown on an uncoated glass surface. These ASC aggregates as well as clusters of ASCs, where formation of the 3D bodies is initiated, expressed pluripotency marker NANOG, but the expression of OCT4A was not detected.
CONCLUSIONS
Obtained results suggest that spontaneously formed ASC aggregates may represent a more primitive cell subpopulation within the individual ASC culture. The ability to form 3D aggregates, the expression of NANOG, and the lack of the AP activity may be used to enrich ASC cultures with potentially more primitive cells serving as an excellent basis for therapeutic applications.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001. 7:211–228.
Article2. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006. 24:1294–1301.
Article3. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006. 24:150–154.
Article4. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. 13:4279–4295.
Article5. Planat-Bénard V, Menard C, André M, Puceat M, Perez A, Garcia-Verdugo JM, Pénicaud L, Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004. 94:223–229.
Article6. Jang S, Cho HH, Cho YB, Park JS, Jeong HS. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010. 11:25.
Article7. Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005. 332:370–379.
Article8. Taléns-Visconti R, Bonora A, Jover R, Mirabet V, Carbonell F, Castell JV, Gómez-Lechón MJ. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol. 2006. 12:5834–5845.
Article9. Chandra V, Swetha G, Muthyala S, Jaiswal AK, Bellare JR, Nair PD, Bhonde RR. Islet-like cell aggregates generated from human adipose tissue derived stem cells ameliorate experimental diabetes in mice. PLoS One. 2011. 6:e20615.
Article10. Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005. 122:947–956.
Article11. Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007. 17:42–49.
Article12. Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005. 26:495–502.
Article13. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006. 99:1285–1297.
Article14. Greco SJ, Liu K, Rameshwar P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells. 2007. 25:3143–3154.
Article15. Lengner CJ, Camargo FD, Hochedlinger K, Welstead GG, Zaidi S, Gokhale S, Scholer HR, Tomilin A, Jaenisch R. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell. 2007. 1:403–415.
Article16. Atlasi Y, Mowla SJ, Ziaee SA, Gokhale PJ, Andrews PW. OCT4 spliced variants are differentially expressed in human pluripotent and nonpluripotent cells. Stem Cells. 2008. 26:3068–3074.
Article17. Booth HA, Holland PW. Eleven daughters of NANOG. Genomics. 2004. 84:229–238.
Article18. Liedtke S, Enczmann J, Waclawczyk S, Wernet P, Kögler G. Oct4 and its pseudogenes confuse stem cell research. Cell Stem Cell. 2007. 1:364–366.
Article19. Lengner CJ, Welstead GG, Jaenisch R. The pluripotency regulator Oct4: a role in somatic stem cells? Cell Cycle. 2008. 7:725–728.
Article20. Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007. 103:389–398.
Article21. Jensen JB, Parmar M. Strengths and limitations of the neurosphere culture system. Mol Neurobiol. 2006. 34:153–161.
Article22. Ivascu A, Kubbies M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J Biomol Screen. 2006. 11:922–932.
Article23. Bartosh TJ, Ylöstalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010. 107:13724–13729.
Article24. Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010. 16:735–749.
Article25. Baraniak PR, McDevitt TC. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multi-lineage potential. Cell Tissue Res. 2012. 347:701–711.
Article26. Cerwinka WH, Sharp SM, Boyan BD, Zhau HE, Chung LWK, Yates C. Differentiation of human mesenchymal stem cell spheroids under microgravity conditions. Cell Regen. 2012. 1:2.
Article27. Sart S, Tsai AC, Li Y, Ma T. Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue Eng Part B Rev. 2014. 20:365–380.
Article28. Van Belle H. Alkaline phosphatase. I. Kinetics and inhibition by levamisole of purified isoenzymes from humans. Clin Chem. 1976. 22:972–976.
Article29. Bogdanova A, Berzins U, Bruvere R, Eivazova G, Kozlovska T. Adipose-derived stem cells cultured in autologous serum maintain the characteristics of mesenchymal stem cells. Proc Latv Acad Sci Sect B Nat Exact Appl Sci. 2010. 64:106–113.
Article30. Bogdanova A, Berzins U, Nikulshin S, Skrastina D, Ezerta A, Legzdina D, Kozlovska T. Characterization of human adipose-derived stem cells cultured in autologous serum after subsequent passaging and long term cryopreservation. J Stem Cells. 2014. 9:135–148.31. Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A, Shigemoto T, Nabeshima Y, Nakahata T, Nabeshima Y, Fujiyoshi Y, Dezawa M. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci U S A. 2010. 107:8639–8643.
Article32. Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013. 11:146.
Article33. Ho M, Yu D, Davidsion MC, Silva GA. Comparison of standard surface chemistries for culturing mesenchymal stem cells prior to neural differentiation. Biomaterials. 2006. 27:4333–4339.
Article34. Lennon DP, Haynesworth SE, Bruder SP, Jaiswal N, Caplan AI. Human and animal mesenchymal progenitor cell from bone marrow: identification of serum for optimal selection and proliferation. In Vitro Cell Dev Biol - Animal. 1996. 32:602–611.
Article35. Butterworth PJ. Alkaline phosphatase. Biochemistry of mammalian alkaline phosphatases. Cell Biochem Funct. 1983. 1:66–70.36. Berstine EG, Hooper ML, Grandchamp S, Ephrussi B. Alkaline phosphatase activity in mouse teratoma. Proc Natl Acad Sci U S A. 1973. 70:3899–3903.
Article37. Millán JL. Alkaline Phosphatases : Structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal. 2006. 2:335–341.38. Kim YH, Yoon DS, Kim HO, Lee JW. Characterization of different subpopulations from bone marrow-derived mesenchymal stromal cells by alkaline phosphatase expression. Stem Cells Dev. 2012. 21:2958–2968.
Article39. Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Schöler H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998. 95:379–391.
Article40. Zuk PA. The intracellular distribution of the ES cell totipotent markers OCT4 and Sox2 in adult stem cells differs dramatically according to commercial antibody used. J Cell Biochem. 2009. 106:867–877.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison with human amniotic membrane- and adipose tissue-derived mesenchymal stem cells
- Mass spectrometry based proteomic analysis of human stem cells: a brief review
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation
- The Rapid Establishment of Human Clonal Adipose Derived Stem Cell (hADSC) Lines with Aspirated Adipose Tissue
- Neuronal Differentiation of a Human Induced Pluripotent Stem Cell Line (FS-1) Derived from Newborn Foreskin Fibroblasts