Korean J Urol.
2014 Jun;55(6):417-425. 10.4111/kju.2014.55.6.417.
Beta-Defensin 124 Is Required for Efficient Innate Immune Responses in Prostate Epithelial RWPE-1 Cells
- Affiliations
-
- 1Research Institute for Translational System Biomics, Chung-Ang University College of Medicine, Seoul, Korea. uromyung@cau.ac.kr
- 2Department of Urology, Hallym University Dontan Sacred Heart Hospital, Hwaseong, Korea.
- 3Department of Urology, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 1885622
- DOI: http://doi.org/10.4111/kju.2014.55.6.417
Abstract
- PURPOSE
The present study aimed to determine the role played by beta-defensin 124 (DEFB124) in the innate immunity of prostate epithelial RWPE-1 cells during bacterial infection.
MATERIALS AND METHODS
The expression of DEFB124 was examined by quantitative real-time polymerase chain reaction (PCR), Western blotting, and immunocytochemistry. Enzyme-linked immunosorbent assays and quantitative real-time PCR were performed to determine the production of cytokines and chemokines. Western blotting and chromatin immunoprecipitation studies were performed to assess the interaction between DEFB124 and nuclear factor-kappa B (NF-kappaB) in peptidoglycan (PGN)-stimulated RWPE-1 cells. By chemotaxis assay, we assessed the effect of DEFB124 on the migration of monocytes.
RESULTS
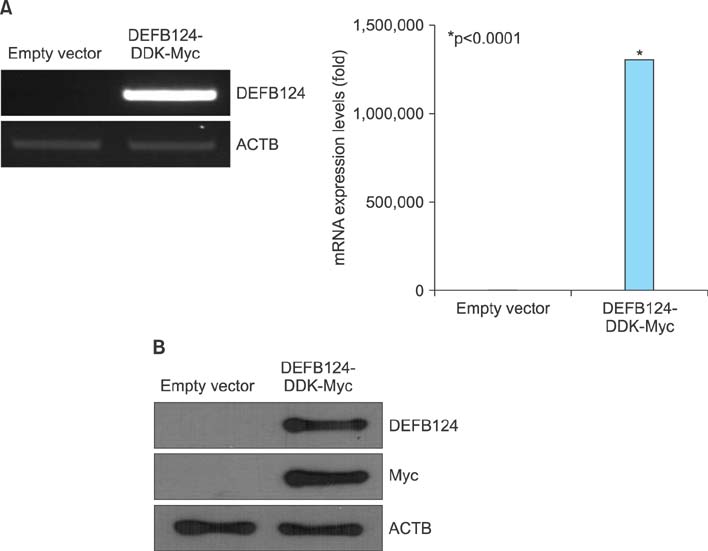
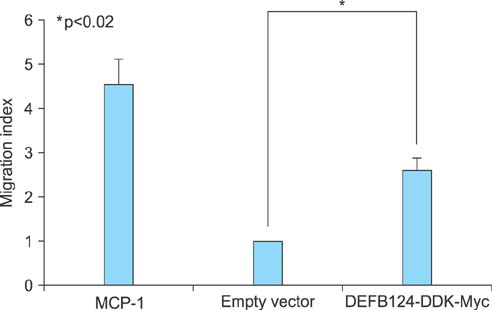
Exposure to PGN induced DEFB124 upregulation and NF-kappaB activation through IkappaBalpha phosphorylation and IkappaBalpha degradation. Bay11-7082, an NF-kappaB inhibitor, blocked PGN-induced DEFB124 production. Also, NF-kappaB was shown to be a direct regulator and to directly bind to the -3.14 kb site of the DEFB124 promoter in PGN-treated human prostate epithelial RWPE-1 cells. When DEFB124 was overexpressed in RWPE-1 cells, interestingly, the production of cytokines (interleukin [IL] 6 and IL-12) and chemokines (CCL5, CCL22, and CXCL8) was significantly increased. These DEFB124-upregulated RWPE-1 cells markedly induced chemotactic activity for THP-1 monocytes.
CONCLUSIONS
Taken together, these results provide strong evidence for the first time that increased DEFB124 expression via NF-kappaB activation in PGN-exposed RWPE-1 cells enhances the production of cytokines and chemokines, which may contribute to an efficient innate immune defense.
Keyword
MeSH Terms
-
Bacterial Infections
Blotting, Western
Chemokines
Chemotaxis
Chromatin Immunoprecipitation
Cytokines
Defensins
Enzyme-Linked Immunosorbent Assay
Humans
Immunity, Innate*
Immunohistochemistry
Monocytes
NF-kappa B
Peptidoglycan
Phosphorylation
Prostate*
Real-Time Polymerase Chain Reaction
Up-Regulation
Chemokines
Cytokines
Defensins
NF-kappa B
Peptidoglycan
Figure
Reference
-
1. Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995; 13:61–92.2. Nizet V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol. 2006; 8:11–26.3. Izadpanah A, Gallo RL. Antimicrobial peptides. J Am Acad Dermatol. 2005; 52(3 Pt 1):381–390.4. Nguyen LT, Haney EF, Vogel HJ. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011; 29:464–472.5. Ganz T. The role of antimicrobial peptides in innate immunity. Integr Comp Biol. 2003; 43:300–304.6. Ganz T. Defensins: antimicrobial peptides of vertebrates. C R Biol. 2004; 327:539–549.7. Tang YQ, Yuan J, Miller CJ, Selsted ME. Isolation, characterization, cDNA cloning, and antimicrobial properties of two distinct subfamilies of alpha-defensins from rhesus macaque leukocytes. Infect Immun. 1999; 67:6139–6144.8. Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009; 30:131–141.9. Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003; 3:710–720.10. Wei G, de Leeuw E, Pazgier M, Yuan W, Zou G, Wang J, et al. Through the looking glass, mechanistic insights from enantiomeric human defensins. J Biol Chem. 2009; 284:29180–29192.11. Schutte BC, Mitros JP, Bartlett JA, Walters JD, Jia HP, Welsh MJ, et al. Discovery of five conserved beta -defensin gene clusters using a computational search strategy. Proc Natl Acad Sci U S A. 2002; 99:2129–2133.12. Bajaj-Elliott M, Fedeli P, Smith GV, Domizio P, Maher L, Ali RS, et al. Modulation of host antimicrobial peptide (beta-defensins 1 and 2) expression during gastritis. Gut. 2002; 51:356–361.13. Motzkus D, Schulz-Maronde S, Heitland A, Schulz A, Forssmann WG, Jubner M, et al. The novel beta-defensin DEFB123 prevents lipopolysaccharide-mediated effects in vitro and in vivo. FASEB J. 2006; 20:1701–1702.14. Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004; 25:280–288.15. Kim HJ, Jung JR, Kim HJ, Lee SY, Chang IH, Lee TJ, et al. Expression of human β-defensin-2 in the prostate. BJU Int. 2011; 107:144–149.16. Andresen L, Jorgensen VL, Perner A, Hansen A, Eugen-Olsen J, Rask-Madsen J. Activation of nuclear factor kappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut. 2005; 54:503–509.17. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009; 1:a001651.18. Wehkamp J, Harder J, Wehkamp K, Wehkamp-von Meissner B, Schlee M, Enders C, et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun. 2004; 72:5750–5758.19. Steubesand N, Kiehne K, Brunke G, Pahl R, Reiss K, Herzig KH, et al. The expression of the beta-defensins hBD-2 and hBD-3 is differentially regulated by NF-kappaB and MAPK/AP-1 pathways in an in vitro model of Candida esophagitis. BMC Immunol. 2009; 10:36.20. Mburu YK, Abe K, Ferris LK, Sarkar SN, Ferris RL. Human β-defensin 3 promotes NF-κB-mediated CCR7 expression and anti-apoptotic signals in squamous cell carcinoma of the head and neck. Carcinogenesis. 2011; 32:168–174.21. Watford WT, Moriguchi M, Morinobu A, O'Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003; 14:361–368.22. Jones LL, Vignali DA. Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunol Res. 2011; 51:5–14.23. Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002; 14:129–135.24. Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002; 71:173–183.25. Amerio P, Frezzolini A, Abeni D, Teofoli P, Girardelli CR, De Pita O, et al. Increased IL-18 in patients with systemic lupus erythematosus: relations with Th-1, Th-2, pro-inflammatory cytokines and disease activity. IL-18 is a marker of disease activity but does not correlate with pro-inflammatory cytokines. Clin Exp Rheumatol. 2002; 20:535–538.26. Maczynska I, Millo B, Ratajczak-Stefanska V, Maleszka R, Szych Z, Kurpisz M, et al. Proinflammatory cytokine (IL-1beta, IL-6, IL-12, IL-18 and TNF-alpha) levels in sera of patients with subacute cutaneous lupus erythematosus (SCLE). Immunol Lett. 2006; 102:79–82.27. Berin MC, Dwinell MB, Eckmann L, Kagnoff MF. Production of MDC/CCL22 by human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001; 280:G1217–G1226.28. Murdoch C, Read RC, Zhang Q, Finn A. Choline-binding protein A of Streptococcus pneumoniae elicits chemokine production and expression of intercellular adhesion molecule 1 (CD54) by human alveolar epithelial cells. J Infect Dis. 2002; 186:1253–1260.29. Kim CH, Broxmeyer HE. Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol. 1999; 65:6–15.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gene Regulatory Regions Required for beta Defensin-2 Up-Regulation by Interleukin-1alpha in the Human Middle Ear Epithelial Cell Line
- Expression of beta Defensins in the Human Middle Ear Mucosa
- Expression of Antimicrobial Defensin Peptides of the Human Nasal Mucosa
- A Dynamic Interplay of Innate Immune Responses During Urinary Tract Infection
- The Innate Immune Responses in Pathogenesis of Chronic Rhinosinusitis