Ann Surg Treat Res.
2014 Aug;87(2):53-60. 10.4174/astr.2014.87.2.53.
Effects of glucose concentration in the medium on rat hepatocyte culture
- Affiliations
-
- 1Department of Surgery, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. kimdg@catholic.ac.kr
- 2Department of Pathology, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 1882831
- DOI: http://doi.org/10.4174/astr.2014.87.2.53
Abstract
- PURPOSE
To determine the optimum culture conditions by investigating isolated rat hepatocytes cultured in medium containing different glucose concentrations.
METHODS
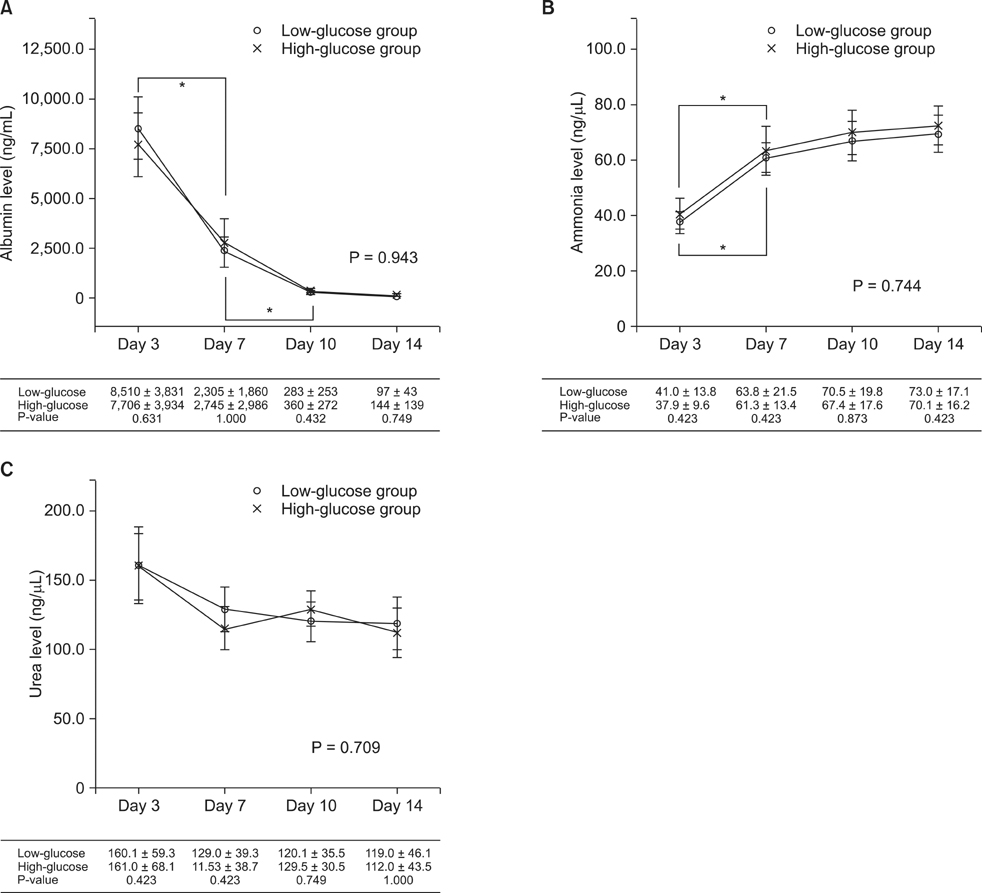
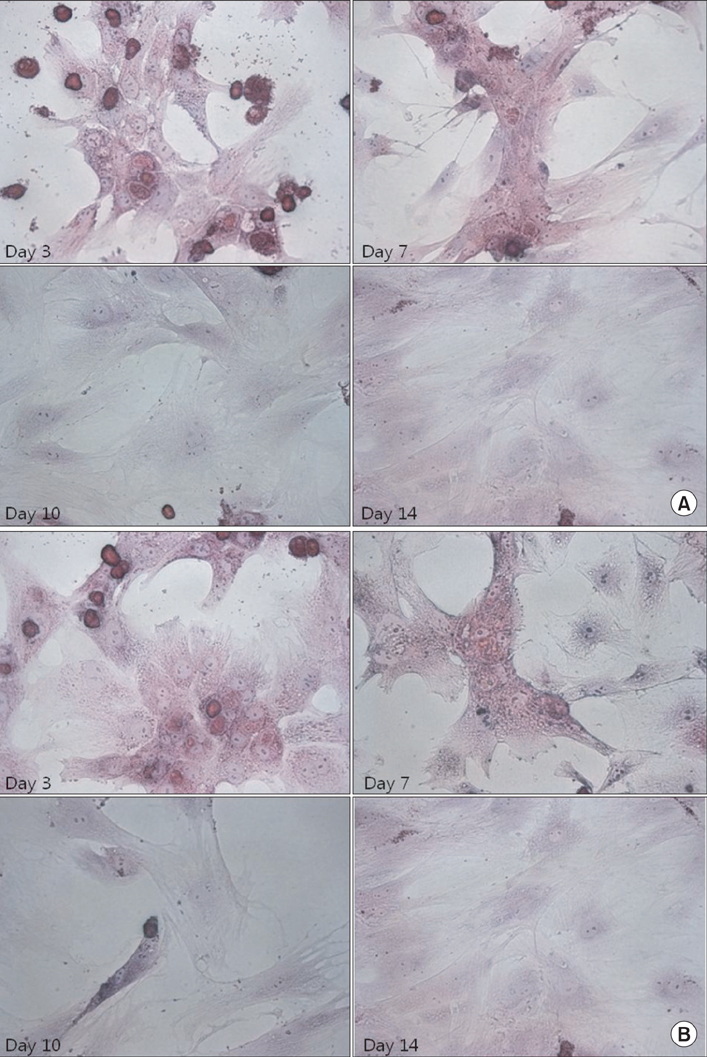
Hepatocytes were isolated from rats using a two-step perfusion technique and divided into the following two groups cultured in medium containing different glucose concentrations: (1) low-glucose group and (2) high-glucose group. Total cell count and viability of cultured rat hepatocytes and liver function parameters (i.e., concentrations of albumin, ammonia, and urea in the culture medium) were measured. The morphology of cultured rat hepatocytes was examined by staining with hematoxylin and eosin, and albumin receptor expression was confirmed by immunofluorescence.
RESULTS
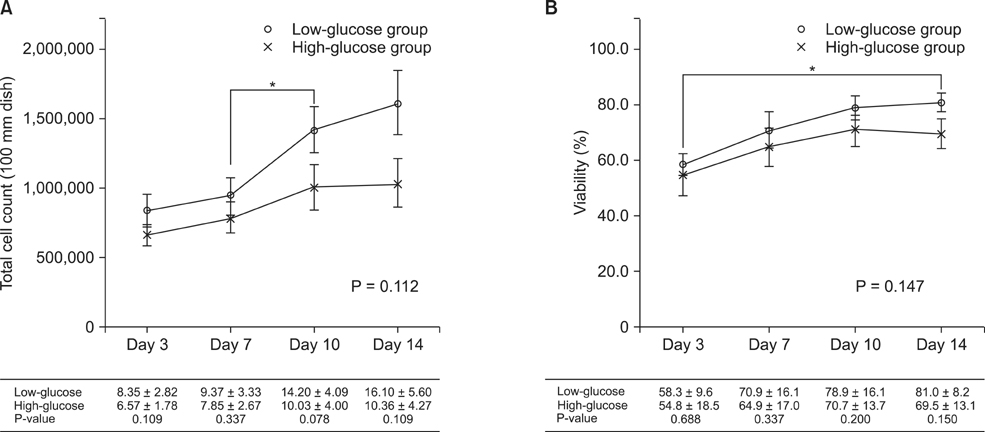
Total cell count and viability showed smaller increases in the low-glucose group than the high-glucose group, although the difference was not statistically significant (P = 0.112 and P = 0.147, respectively). The levels of albumin (P = 0.943), ammonia (P = 0.744), and urea (P = 0.709) were not significantly different between the two groups. In both groups, the function of cultured hepatocytes decreased significantly over time. The morphology of hepatocytes was well maintained in both groups at 3 days. On day 7, the cytoplasm was transformed into a spindle shape. On day 10, these changes were exaggerated, and were more prominent in the high-glucose group.
CONCLUSION
Morphological assessment indicated that low-glucose culture medium is better than high-glucose culture medium for culturing of hepatocytes, although there was not significantly different in functional assessment. The cultured hepatocytes with low-glucose culture medium could be maintained for 7 days.
Keyword
MeSH Terms
Figure
Reference
-
1. Gotthardt D, Riediger C, Weiss KH, Encke J, Schemmer P, Schmidt J, et al. Fulminant hepatic failure: etiology and indications for liver transplantation. Nephrol Dial Transplant. 2007; 22:Suppl 8. viii5–viii8.2. Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA. 2003; 289:217–222.3. Demetriou AA, Brown RS Jr, Busuttil RW, Fair J, McGuire BM, Rosenthal P, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004; 239:660–667.4. Gill RQ, Sterling RK. Acute liver failure. J Clin Gastroenterol. 2001; 33:191–198.5. Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010; 7:288–298.6. Hughes RD, Mitry RR, Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012; 93:342–347.7. Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976; 13:29–83.8. Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, et al. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976; 192:892–894.9. Mito M, Kusano M, Kawaura Y. Hepatocyte transplantation in man. Transplant Proc. 1992; 24:3052–3053.10. Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998; 338:1422–1426.11. Howard RB, Christensen AK, Gibbs FA, Pesch LA. The enzymatic preparation of isolated intact parenchymal cells from rat liver. J Cell Biol. 1967; 35:675–684.12. Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969; 43:506–520.13. Hughes RD, Mitry RR, Dhawan A. Hepatocyte transplantation for metabolic liver disease: UK experience. J R Soc Med. 2005; 98:341–345.14. Nyberg SL, Peshwa MV, Payne WD, Hu WS, Cerra FB. Evolution of the bioartificial liver: the need for randomized clinical trials. Am J Surg. 1993; 166:512–521.15. Hayashi I, Sato GH. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. 1976; 259:132–134.16. Matsushita T, Ijima H, Koide N, Funatsu K. High albumin production by multicellular spheroids of adult rat hepatocytes formed in the pores of polyurethane foam. Appl Microbiol Biotechnol. 1991; 36:324–326.17. Guillouzo A, Beaune P, Gascoin MN, Begue JM, Campion JP, Guengerich FP, et al. Maintenance of cytochrome P-450 in cultured adult human hepatocytes. Biochem Pharmacol. 1985; 34:2991–2995.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cryopreservation of Rat Hepatocytes for the Use of Primary Culture
- The Effect of Doxycycline on the Survival of the Cells under Hypoxic Condition
- Successful mouse hepatocyte culture with sandwich collagen gel formation
- Hepatocyte Lacks Insulin-Mediated Glucose Transporter Translocation Mechanism in Rat
- A Study of Human Melanocytes Culture