Tuberc Respir Dis.
2007 Jul;63(1):42-51. 10.4046/trd.2007.63.1.42.
Enhancement of Sensitivity of Human Lung Cancer Cell Line to TRAIL and Gefitinib by IGF-1R Blockade
- Affiliations
-
- 1Department of Internal Medicine, Lung Institute of Medical Research Center, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Medicine, Respiratory Center, Seoul National University Bundang Hospital, Seongnam, Korea. ctlee@snu.ac.kr
- KMID: 1877316
- DOI: http://doi.org/10.4046/trd.2007.63.1.42
Abstract
-
BACKGROUND: TRAIL is a cytokine that selectively induces apoptosis in various cancer cell lines. Gefitinib is new targeted drug applied in lung cancer that selectively inhibits EGFR tyrosine kinase. However, lung cancers have shown an initial or acquired resistance to these drugs. This study examined the effect of IGF-1R and its blockade on enhancing the sensitivity of lung cancer cell lines to TRAIL and gefitinib.
METHODS
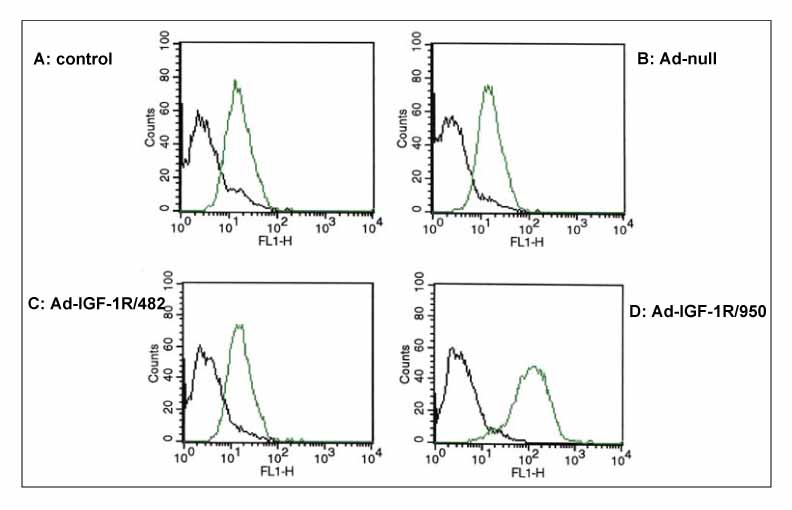
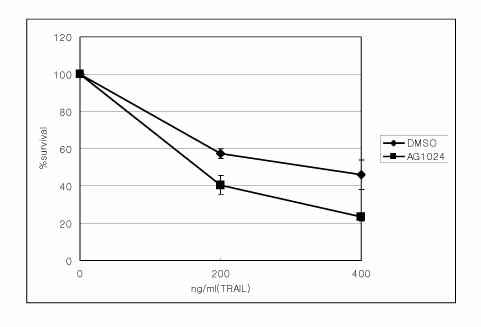
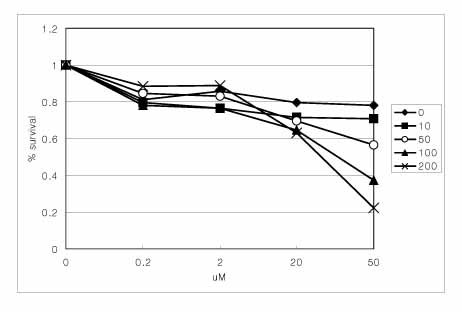
Two lung cancer cell lines were used in this study. NCI H460 is very sensitive to TRAIL and gefitinib. On the other hand, A549 shows moderate resistance to TRAIL and gefitinib. The IGF-1R blockade was performed using adenoviruses expressing the dominant negative IGF-1R and shRNA to IGF-1R and AG1024 (IGF-1R tyrosine kinase inhibitor).
RESULTS
The adenovirus expressing dominant negative IGF-1R(950st) induced the increased expression of defective IGF-1R on the lung cancer cell surface, and the adenovirus-shIGF-1R effectively decreased the level of IGF-1R expression on cell surface. The genetic blockade of IGF-1R by the adenovirus-dnIGF-1R and AG1024 increased the sensitivity of A549 cells to TRAIL. The reduction of IGF-1R by transduction with ad-shIGF-1R also increased the sensitivity of the A549 cells to gefitinib.
CONCLUSION
The blockade of IGF-1R through various mechanisms increased the sensitivity of the lung cancer cell line that was resistant to TRAIL and gefitinib. However, further studies using other cell lines showing acquired resistance as well as in vivo animal experiments will be needed.
Keyword
MeSH Terms
Figure
Reference
-
1. Griffith TS, Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998. 10:559–563.2. Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005. 12:228–237.3. Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997. 277:815–818.4. Lee SH, Shin MS, Kim HS, Lee HK, Park WS, Kim SY, et al. Alterations of the DR5/TRAIL receptor 2 gene in non-small cell lung cancers. Cancer Res. 1999. 59:5683–5686.5. Cheng J, Hylander BL, Baer MR, Chen X, Repasky EA. Multiple mechanisms underlie resistance of leukemia cells to Apo2 Ligand/TRAIL. Mol Cancer Ther. 2006. 5:1844–1853.6. Brooks AD, Sayers TJ. Reduction of the antiapoptotic protein cFLIP enhances the susceptibility of human renal cancer cells to TRAIL apoptosis. Cancer Immunol Immunother. 2005. 54:499–505.7. Seol JY, Park KH, Hwang CI, Park WY, Yoo CG, Kim YW, et al. Adenovirus-TRAIL can overcome TRAIL resistance and induce a bystander effect. Cancer Gene Ther. 2003. 10:540–548.8. Herbst RS, Maddox AM, Rothenberg ML, Small EJ, Rubin EH, Baselga J, et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol. 2002. 20:3815–3825.9. Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced nonsmall-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol. 2004. 22:785–794.10. Lee DH, Han JY, Kim HT, Lee JS. Gefitinib is of more benefit in chemotherapy-naive patients with good performance status and adenocarcinoma histology: retrospective analysis of 575 Korean patients. Lung Cancer. 2006. 53:339–345.11. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004. 350:2129–2139.12. Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005. 23:2493–2501.13. Lee CT, Adachi Y, Carbone DP. IGF-1R blockade strategies in human cancers. Gene Ther Mol Biol. 2005. 9:77–88.14. Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997. 17:1595–1606.15. Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997. 91:231–241.16. Poulaki V, Mitsiades CS, Kotoula V, Tseleni-Balafouta S, Ashkenazi A, Koutras DA, et al. Regulation of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in thyroid carcinoma cells. Am J Pathol. 2002. 161:643–654.17. Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, et al. Activation of NF-kappaB and upregulation of intracellular antiapoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002. 21:5673–5683.18. Jones HE, Goddard L, Gee JM, Hiscox S, Rubini M, Barrow D, et al. Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer. 2004. 11:793–814.19. Camirand A, Zakikhani M, Young F, Pollak M. Inhibition of insulin-like growth factor-1 receptor signaling enhances growth-inhibitory and proapoptotic effects of gefitinib (Iressa) in human breast cancer cells. Breast Cancer Res. 2005. 7:R570–R579.20. Desbois-Mouthon C, Cacheux W, Blivet-Van Eggelpoel MJ, Barbu V, Fartoux L, Poupon R, et al. Impact of IGF-1R/EGFR cross-talks on hepatoma cell sensitivity to gefitinib. Int J Cancer. 2006. 119:2557–2566.21. Lee CT, Park KH, Adachi Y, Seol JY, Yoo CG, Kim YW, et al. Recombinant adenoviruses expressing dominant negative insulin-like growth factor-I receptor demonstrate antitumor effects on lung cancer. Cancer Gene Ther. 2003. 10:57–63.22. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998. 391:806–811.23. Lee YJ, Imsumran A, Park MY, Kwon SY, Yoon HI, Lee JH, et al. Adenovirus expressing shRNA to IGF-1R enhances the chemosensitivity of lung cancer cell lines by blocking IGF-1 pathway. Lung Cancer. 2007. 55:279–286.24. Lee CT, Carbone DP. Pass HI, Carbone DP, Johnson DH, Minna JD, Turrisi AT, editors. Chapter 52. Gene Therapy. Lung Cancer. 2005. Philadelphia: Lippincott Williams & Wilkins;734–753.25. Parrizas M, Gazit A, Levitzki A, Wertheimer E, LeRoith D. Specific inhibition of insulin-like growth factor-1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins. Endocrinology. 1997. 138:1427–1433.26. Karacay B, Sanlioglu S, Griffith TS, Sandler A, Bonthius DJ. Inhibition of the NF-kappaB pathway enhances TRAIL-mediated apoptosis in neuroblastoma cells. Cancer Gene Ther. 2004. 11:681–690.27. Franco AV, Zhang XD, Van Berkel E, Sanders JE, Zhang XY, Thomas WD, et al. The role of NF-kappa B in TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis of melanoma cells. J Immunol. 2001. 166:5337–5345.28. Karacay B, Sanlioglu S, Griffith TS, Sandler A, Bonthius DJ. Inhibition of the NF-κB pathway enhances TRAIL-mediated apoptosis in neuroblastoma cells. Cancer Gene Ther. 2004. 11:681–690.29. Adachi Y, Lee CT, Carbone DP. Genetic blockade of the insulin-like growth factor 1 receptor for human malignancy. Novartis Found Symp. 2004. 262:177–189.30. Lee CT, Wu S, Gabrilovich D, Chen H, Nadaf-Rahrov S, Ciernik IF, et al. Antitumor effects of an adenovirus expressing antisense insulin-like growth factor I receptor on human lung cancer cell lines. Cancer Res. 1996. 56:3038–3041.31. Adachi Y, Lee CT, Coffee K, Yamagata N, Ohm JE, Park KH, et al. Effects of genetic blockade of the insulin-like growth factor receptor in human colon cancer cell lines. Gastroenterology. 2002. 123:1191–1204.32. Min Y, Adachi Y, Yamamoto H, Ito H, Itoh F, Lee CT, et al. Genetic blockade of the insulin-like growth factor-I receptor: a promising strategy for human pancreatic cancer. Cancer Res. 2003. 63:6432–6441.33. Morgillo F, Kim WY, Kim ES, Ciardiello F, Hong WK, Lee HY. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res. 2007. 13:2795–2803.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical significance of insulin-like growth factor-1 receptor expression in stage I non-small-cell lung cancer: immunohistochemical analysis
- Comparison of Gefitinib versus Docetaxel in Patients with Pre-Treated Non-Small Cell Lung Cancer (NSCLC)
- Insulin-Like Growth Factor-1 Receptor Targeted Fluorescent Imaging for Gallbladder Cancer in Orthotopic Mouse Models
- The Effectiveness of Gefitinib on Spinal Metastases of Lung Cancer: Report of Two Cases
- A case of leptomeningeal metastasis from adenocarcinoma of the lung improved by treatment with Gefitinib