Korean Circ J.
2007 Feb;37(2):78-83. 10.4070/kcj.2007.37.2.78.
A Slight Variation in the Age of Rats Commonly used as a Carotid Artery Injury Model Results in a Large Difference in Neointima Formation
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Chungbuk National University, Cheongju, Korea. kdwoon@chungbuk.ac.kr
- KMID: 1859085
- DOI: http://doi.org/10.4070/kcj.2007.37.2.78
Abstract
-
BACKGROUND AND OBJECTIVES: The degree of neointima formation after infliction of a carotid artery balloon injury in rats varies greatly depending on the sex, age, species and operational method. Strong variation is common, even within only a single control. This study attempted to find if there was any significant difference in neointima formation following a carotid artery balloon injury in 6 to 12 week old rats; the age commonly used in these types of experiments.
MATERIALS AND METHODS
A balloon injury was inflicted on the carotid arteries of male SpragueDawley rats at 6 (n=9, 250-270 g), 8 (n=8, 280-300 g) and 11 weeks (n=10, 320-340 g) of age. Two weeks postoperation, a histomorphometric analysis was carried out. The vascular smooth muscle cell proliferation was measured in situ via BrdU incorporation 2 days after injury infliction.
RESULTS
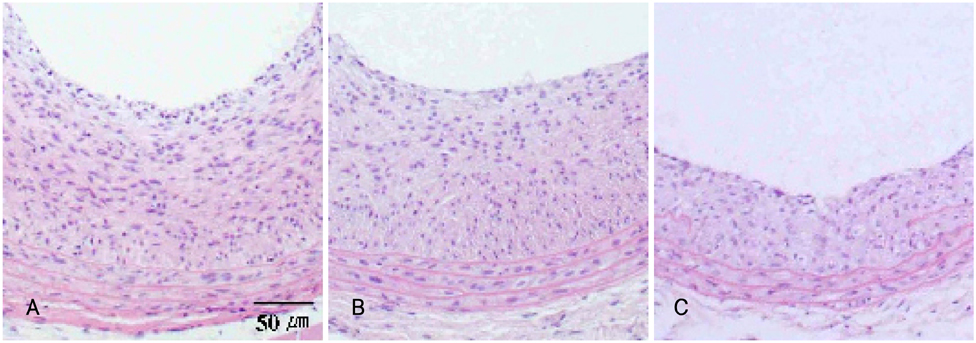
The neointima areas of the 6 week (0.22+/-0.04 mm2) and 8 week old groups (0.17+/-0.08 mm2) were 3.1 and 2.4 times larger than that of the 11 week old group (0.07+/-0.03 mm2). The mitotic index was significantly reduced in 11 week old group (n=4, 9.22+/-1.51%) compared to those of the 6 (n=4, 25.03+/-3.92%) and 8 week old (n=4, 21.66+/-3.66%) groups.
CONCLUSION
Special care should be taken when interpreting neointima formation, as even a slight variation in the age and weight in 6 to 12 week old (250-340 g) rats; the age commonly used in these types of experiments, results in an unexpectedly large difference.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Analysis of Reclaiming Ratio for 3 Diatom Species from Experimentally Drowned Animal Organs
Jeong-Won Hong, Youn Shin Kim
Korean J Leg Med. 2013;37(1):19-26. doi: 10.7580/kjlm.2013.37.1.19.
Reference
-
1. Kantor B, Ashai K, Holmes DR Jr, Schwartz RS. The experimental animal models for assessing treatment of restenosis. Cardiovasc Radiat Med. 1999. 1:48–54.2. Schwartz RS. Neointima and arterial injury: dogs, rats, pigs and more. Lab Invest. 1994. 71:789–791.3. Touchard AG, Schwartz RS. Preclinical restenosis models: challenges and successes. Toxicol Pathol. 2006. 34:11–18.4. Ferns GA, Avades TY. The mechanism of coronary restenosis: insights from experimental models. Int J Exp Pathol. 2000. 81:63–88.5. Fuster V, Ip JH, Badimon L, Badimon JJ, Stein B, Chesebro JH. Importance of experimental models for the development of clinical trials on thromboatherosclerosis. Circulation. 1991. 83:Suppl. IV15–IV25.6. Folts J. An in vivo model of experimental arterial stenosis, intimal damage and periodic thrombosis. Circulation. 1991. 83:Suppl. IV3–IV14.7. Assandnia S, Rapp JP, Nestor AL, et al. Strain difference in neointimal hyperplasia in the rat. Circ Res. 1999. 84:1252–1257.8. Spagnoli LG, Sambuy Y, Palmieri G, Mauriello A. Age related modulation of vascular smooth muscle cells proliferation following arterial wall damage. Artery. 1985. 13:187–198.9. Chajara A, Delpech B, Courel MN, Leroy M, Basuyau JP, Levesque H. Effect of aging on neointima formation and hyaluronan, hyaluronidase and hyaluronectin production in injured rat aorta. Atherosclerosis. 1998. 138:53–64.10. McCaffrey TA, Nicholson AC, Szabo PE, Weksler ME, Weksler BB. Aging and arteriosclerosis: the increased proliferation of arterial smooth muscle cells isolated from old rats is associated with increased platelet-derived growth factor-like activity. J Exp Med. 1988. 167:163–174.11. Stemerman MB, Weinstein R, Rowe JW, Maciag T, Fuhro R, Gardner R. Vascular smooth muscle cells growth kinetics in vivo in aged rats. Proc Natl Acad Sci U S A. 1982. 79:3863–3866.12. Hariri RJ, Alonso DR, Hajjar DP, Coletti D, Weksler ME. Aging and arteriosclerosis: I. development of myointimal hyperplasia after endothelial injury. J Exp Med. 1986. 164:1171–1178.13. Kwon JS, Park SS, Kim YG, et al. Perivascular delivery of paclitaxel with F-127 pluronic gel inhibits neointimal hyperplasia in a rat carotid artery injury model. Korean Circ J. 2005. 35:221–227.14. Kim DW, Kwon JS, Kim YG, et al. Novel oral formulation of paclitaxel inhibits neointimal hyperplasia in a rat carotid artery injury model. Circulation. 2004. 109:1558–1563.15. Cho MC, Kwak NJ, Piao HN, et al. Effect of paclitaxel local delivery on neointimal formation after endothelial denudation of the rat carotid artery. Korean Circ J. 2000. 30:198–207.16. Kim DW, Kim YG, Oh TG, Cho MC, Kim ST. Retrovirus-mediated herpes simplex virus thymidine kinase gene therapy for the prevention of stenosis in rat carotid artery injury model. Korean Circ J. 1998. 28:977–989.17. Kim ST, Kim DW, Oh TG, Ahn HY, Kim YG. Gene therapy using retroviral vector containing rat erythropoietin. Korean J Hematol. 1997. 32:22–31.18. Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury: I. smooth muscle growth in the absence of endothelium. Lab Invest. 1983. 49:327–333.19. Sollott SJ, Cheng L, Pauly RR, et al. Taxol inhibits neointimal smooth muscle cell accumulation after angioplasty in the rat. J Clin Invest. 1995. 95:1869–1876.20. Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992. 19:267–274.21. Harman SM. Testosterone in older men after the Institute of Medicine Report: where do we go from here? Climacteric. 2005. 8:124–135.22. Khaw KT, Barrett-Connor E. Endogenous sex hormones, high density lipoprotein cholesterol, and other lipoprotein fractions in men. Arterioscler Thromb. 1991. 11:489–494.23. Heller RF, Wheeler MJ, Micallef J, Miller NE, Lewis B. Relationship of high density lipoprotein cholesterol with total and free testosterone and sex hormone binding globulin. Acta Endocrinl. 1983. 104:253–256.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of L-arginine on Neointima Formation in a Rat Vascular Injury Model
- The change in phospholipase C-gamma1 expression following balloon injury to the rat carotid artery
- Bortezomib Reduces Neointimal Hyperplasia in a Rat Carotid Artery Injury Model
- Oral Everolimus Reduces Adventitial Cell Activation and Neointima Formation in Balloon-Injured Rat Carotid Artery
- Effect of Paclitaxel Local Delivery on Neointimal Formation after Endothelial Denudation of the Rat Carotid Artery