Tuberc Respir Dis.
2012 Dec;73(6):303-311. 10.4046/trd.2012.73.6.303.
Gefitinib in Selected Patients with Pre-Treated Non-Small-Cell Lung Cancer: Results from a Phase IV, Multicenter, Non-Randomized Study (SELINE)
- Affiliations
-
- 1Department of Internal Medicine, Yeungnam University Medical Center, Yeungnam University College of Medicine, Daegu, Korea. ghlee@med.yu.ac.kr
- 2Department of Internal Medicine, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea.
- 4Department of Internal Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea.
- 5Department of Internal Medicine, Dong-A University Medical Center, Dong-A University College of Medicine, Busan, Korea.
- 6Department of Internal Medicine, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea.
- 7Department of Internal Medicine, Inha University Hospital, Inha University School of Medicine, Incheon, Korea.
- 8Department of Internal Medicine, Wonkwang University Hospital, Wonkwang University School of Medicine, Iksan, Korea.
- 9Department of Internal Medicine, Universitivy of Ulsan College of Medicine, Seoul, Korea.
- 10Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 11Department of Internal Medicine, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea.
- KMID: 1842923
- DOI: http://doi.org/10.4046/trd.2012.73.6.303
Abstract
- BACKGROUND
This study was designed to analyze the efficacy of gefitinib as a second-line therapy, according to the clinical characteristics in Korean patients with non-small-cell lung cancer (NSCLC).
METHODS
In this Phase IV observational study, we recruited patients, previously failed first-line chemotherapy, who had locally advanced or metastatic NSCLC, and who were found to be either epidermal growth factor receptor (EGFR) mutation-positive or satisfied 2 or more of the 3 characteristics: adenocarcinoma, female, and non-smoker. These patients were administered with gefitinib 250 mg/day, orally. The primary endpoints were to evaluate the objective response rate (ORR) and to determine the relationship of ORRs, depending on each patient's characteristics of modified intent-to-treat population.
RESULTS
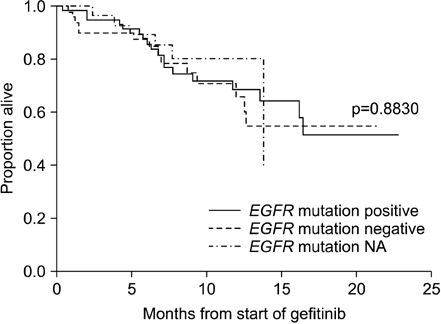
A total of 138 patients participated in this study. One subject achieved complete response, and 42 subjects achieved partial response (ORR, 31.2%). The subgroup analysis demonstrated that the ORR was significantly higher in patients with EGFR mutation-positive, compared to that of EGFR mutation-negative (45.8% vs. 14.0%, p=0.0004). In a secondary efficacy variable, the median progression-free survival (PFS) was 5.7 months (95% confidence interval, 3.9~8.4 months) and the 6-month PFS and overall survival were 49.6% and 87.9%, respectively. The most common reported adverse events were rash (34.4%), diarrhea (26.6%), pruritus (17.5%), and cough (15.6%).
CONCLUSION
Gefitinib was observed in anti-tumor activity with favorable tolerability profile as a second-line therapy in these selected patients. When looking at EGFR mutation status, EGFR mutation-positive showed strong association with gefitinib by greater response and prolonged PFS, compared with that of EGFR mutation-negative.
Keyword
MeSH Terms
Figure
Reference
-
1. Jang TW, Oak CH, Chang HK, Suo SJ, Jung MH. EGFR and KRAS mutations in patients with adenocarcinoma of the lung. Korean J Intern Med. 2009. 24:48–54.2. Zhang XT, Li LY, Mu XL, Cui QC, Chang XY, Song W, et al. The EGFR mutation and its correlation with response of gefitinib in previously treated Chinese patients with advanced non-small-cell lung cancer. Ann Oncol. 2005. 16:1334–1342.3. Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005. 23:2493–2501.4. Han SW, Hwang PG, Chung DH, Kim DW, Im SA, Kim YT, et al. Epidermal growth factor receptor (EGFR) downstream molecules as response predictive markers for gefitinib (Iressa, ZD1839) in chemotherapy-resistant non-small cell lung cancer. Int J Cancer. 2005. 113:109–115.5. Hong J, Kyung SY, Lee SP, Park JW, Jung SH, Lee JI, et al. Pemetrexed versus gefitinib versus erlotinib in previously treated patients with non-small cell lung cancer. Korean J Intern Med. 2010. 25:294–300.6. Park K, Goto K. A review of the benefit-risk profile of gefitinib in Asian patients with advanced non-small-cell lung cancer. Curr Med Res Opin. 2006. 22:561–573.7. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005. 366:1527–1537.8. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004. 350:2129–2139.9. Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004. 304:1497–1500.10. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004. 101:13306–13311.11. Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011. 29:2866–2874.12. Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008. 372:1809–1818.13. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.14. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003. 21:2237–2246.15. Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003. 290:2149–2158.16. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003. 8:303–306.17. Wu JY, Yu CJ, Yang CH, Wu SG, Chiu YH, Gow CH, et al. First- or second-line therapy with gefitinib produces equal survival in non-small cell lung cancer. Am J Respir Crit Care Med. 2008. 178:847–853.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Gefitinib versus Docetaxel in Patients with Pre-Treated Non-Small Cell Lung Cancer (NSCLC)
- A case of leptomeningeal metastasis from adenocarcinoma of the lung improved by treatment with Gefitinib
- The Effectiveness of Gefitinib on Spinal Metastases of Lung Cancer: Report of Two Cases
- Acute Respiratory Failure Developed in Non-small Cell Lung Cancer Patients Treated With Gefitinib
- Overcoming the Intrinsic Gefitinib-resistance via Downregulation of AXL in Non-small Cell Lung Cancer