Korean J Urol.
2013 Aug;54(8):492-498. 10.4111/kju.2013.54.8.492.
Current Evaluation and Treatment of Nocturia
- Affiliations
-
- 1Department of Urology, Konkuk University School of Medicine, Seoul, Korea. khgsjh@kuh.ac.kr
- KMID: 1840459
- DOI: http://doi.org/10.4111/kju.2013.54.8.492
Abstract
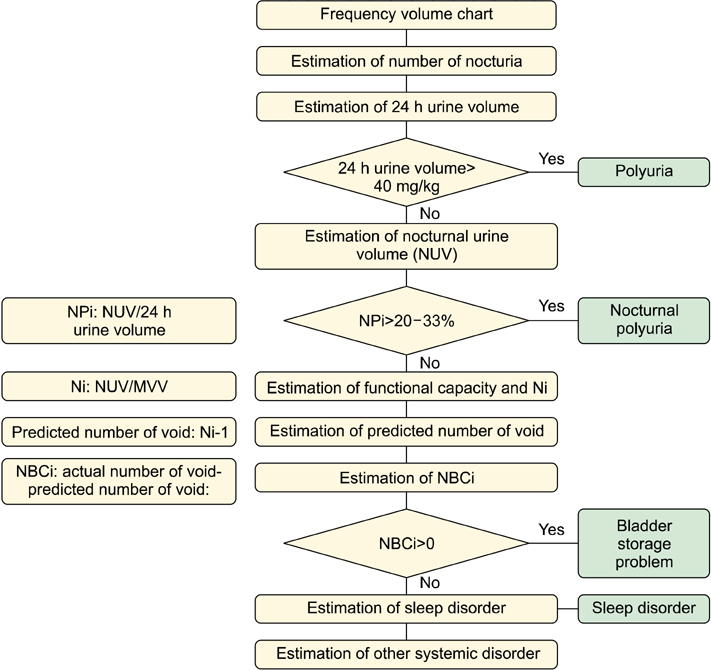
- Nocturia is usually considered to be just one of the symptoms included with lower urinary tract symptoms (LUTS) and is treated with therapy based on LUTS. Recent research suggests, however, that nocturia is not merely a simple symptom of LUTS but is a multifactorial condition with many contributing etiological factors. The causes of nocturia can be classified into bladder storage problems, increased urine output, sleep disturbance problems, and other potential diseases. The frequency-volume chart (FVC) is very important in evaluating and diagnosing nocturia. Patients usually record the volume and timing of voids for a period of 1 to 3 days on the FVC. The FVC data can provide information on voiding patterns and clues about the etiology and treatment of nocturia. It is doubtful that alpha-blockers will have clinical significance for treatment because the difference in nocturia episodes between treatment with alpha-blockers and placebo is too small. Antimuscarinics also exert no effect on nocturnal polyuria, and the evidence supporting the efficacy of antimuscarinics for the treatment of nocturia is limited. However, several randomized placebo-controlled trials have shown the efficacy of oral desmopressin in the treatment of adults with nocturia. Short-acting hypnotics may be helpful for patients with sleep disturbances. Although surgical or interventional therapy is not indicated for nocturia, transurethral resection of the prostate appears to confer a greater improvement in benign prostatic hyperplasia symptoms including nocturia. The management of nocturia may require a team approach by making optimal use of multidisciplinary expertise.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Is It Worth Treating Non-Bothering Nocturia? Results of a Multicenter Prospective Observational Study
Kwangsung Park, Hyoung Keun Park, Sae Woong Kim, Dae Yul Yang, Jong Kwan Park, Hyun Jun Park, Ki Hak Moon, Du Geon Moon, Kweon Sik Min, Hwancheol Son, Sung Won Lee, Jae Seog Hyun, Woo Suk Choi, Sang-Kuk Yang
World J Mens Health. 2018;36(3):248-254. doi: 10.5534/wjmh.170003.
Reference
-
1. van Kerrebroeck P, Abrams P, Chaikin D, Donovan J, Fonda D, Jackson S, et al. The standardisation of terminology in nocturia: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002. 21:179–183.2. Weiss JP, Blaivas JG, Bliwise DL, Dmochowski RR, Dubeau CE, Lowe FC, et al. The evaluation and treatment of nocturia: a consensus statement. BJU Int. 2011. 108:6–21.3. Coyne KS, Zhou Z, Bhattacharyya SK, Thompson CL, Dhawan R, Versi E. The prevalence of nocturia and its effect on health-related quality of life and sleep in a community sample in the USA. BJU Int. 2003. 92:948–954.4. Tikkinen KA, Johnson TM 2nd, Tammela TL, Sintonen H, Haukka J, Huhtala H, et al. Nocturia frequency, bother, and quality of life: how often is too often? A population-based study in Finland. Eur Urol. 2010. 57:488–496.5. Fitzgerald MP, Litman HJ, Link CL, McKinlay JB. BACH Survey Investigators. The association of nocturia with cardiac disease, diabetes, body mass index, age and diuretic use: results from the BACH survey. J Urol. 2007. 177:1385–1389.6. Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006. 50:1306–1314.7. Bosch JL, Weiss JP. The prevalence and causes of nocturia. J Urol. 2010. 184:440–446.8. Hunter DJ, Berra-Unamuno A, Martin-Gordo A. Prevalence of urinary symptoms and other urological conditions in Spanish men 50 years old or older. J Urol. 1996. 155:1965–1970.9. Rembratt A, Norgaard JP, Andersson KE. Differences between nocturics and non-nocturics in voiding patterns: an analysis of frequency-volume charts from community-dwelling elderly. BJU Int. 2003. 91:45–50.10. Bing MH, Moller LA, Jennum P, Mortensen S, Skovgaard LT, Lose G. Prevalence and bother of nocturia, and causes of sleep interruption in a Danish population of men and women aged 60-80 years. BJU Int. 2006. 98:599–604.11. Lukacz ES, Whitcomb EL, Lawrence JM, Nager CW, Luber KM. Urinary frequency in community-dwelling women: what is normal? Am J Obstet Gynecol. 2009. 200:552.e1–552.e7.12. Endeshaw YW, Johnson TM, Kutner MH, Ouslander JG, Bliwise DL. Sleep-disordered breathing and nocturia in older adults. J Am Geriatr Soc. 2004. 52:957–960.13. Margel D, Shochat T, Getzler O, Livne PM, Pillar G. Continuous positive airway pressure reduces nocturia in patients with obstructive sleep apnea. Urology. 2006. 67:974–977.14. Kang SH, Yoon IY, Lee SD, Kim JW. The impact of sleep apnoea syndrome on nocturia according to age in men. BJU Int. 2012. 110(11 Pt C):E851–E856.15. Yalkut D, Lee LY, Grider J, Jorgensen M, Jackson B, Ott C. Mechanism of atrial natriuretic peptide release with increased inspiratory resistance. J Lab Clin Med. 1996. 128:322–328.16. Cornu JN, Abrams P, Chapple CR, Dmochowski RR, Lemack GE, Michel MC, et al. A contemporary assessment of nocturia: definition, epidemiology, pathophysiology, and management: a systematic review and meta-analysis. Eur Urol. 2012. 62:877–890.17. Cho SY, Lee SL, Kim IS, Koo DH, Kim HJ, Oh SJ. Short-term effects of systematized behavioral modification program for nocturia: a prospective study. Neurourol Urodyn. 2012. 31:64–68.18. Speakman M. Efficacy and safety of Tamsulosin OCAS. BJU Int. 2006. 98:Suppl 2. 13–17.19. Djavan B, Milani S, Davies J, Bolodeoku J. The impact of tamsulosin oral controlled absorption system (OCAS) on nocturia and the quality of sleep: preliminary results of a pilot study. Eur Urol Suppl. 2005. 4:61–68.20. Johnson TM 2nd, Jones K, Williford WO, Kutner MH, Issa MM, Lepor H. Changes in nocturia from medical treatment of benign prostatic hyperplasia: secondary analysis of the Department of Veterans Affairs Cooperative Study Trial. J Urol. 2003. 170:145–148.21. Nishino Y, Masue T, Miwa K, Takahashi Y, Ishihara S, Deguchi T. Comparison of two alpha1-adrenoceptor antagonists, naftopidil and tamsulosin hydrochloride, in the treatment of lower urinary tract symptoms with benign prostatic hyperplasia: a randomized crossover study. BJU Int. 2006. 97:747–751.22. Brubaker L, FitzGerald MP. Nocturnal polyuria and nocturia relief in patients treated with solifenacin for overactive bladder symptoms. Int Urogynecol J Pelvic Floor Dysfunct. 2007. 18:737–741.23. Rudy D, Cline K, Harris R, Goldberg K, Dmochowski R. Multicenter phase III trial studying trospium chloride in patients with overactive bladder. Urology. 2006. 67:275–280.24. Rackley R, Weiss JP, Rovner ES, Wang JT, Guan Z. 037 STUDY GROUP. Nighttime dosing with tolterodine reduces overactive bladder-related nocturnal micturitions in patients with overactive bladder and nocturia. Urology. 2006. 67:731–736.25. Herschorn S, Swift S, Guan Z, Carlsson M, Morrow JD, Brodsky M, et al. Comparison of fesoterodine and tolterodine extended release for the treatment of overactive bladder: a head-to-head placebo-controlled trial. BJU Int. 2010. 105:58–66.26. Reynard JM, Cannon A, Yang Q, Abrams P. A novel therapy for nocturnal polyuria: a double-blind randomized trial of frusemide against placebo. Br J Urol. 1998. 81:215–218.27. Cho MC, Ku JH, Paick JS. Alpha-blocker plus diuretic combination therapy as second-line treatment for nocturia in men with LUTS: a pilot study. Urology. 2009. 73:549–553.28. Andersson KE, Chapple CR, Cardozo L, Cruz F, Hashim H, Michel MC, et al. Abrams P, Cardozo L, Khoury S, Wein AJ, editors. Pharmacological treatment of urinary incontinence. Incontinence. 2009. Paris: Health Publication Ltd;631–699.29. Mattiasson A, Abrams P, Van Kerrebroeck P, Walter S, Weiss J. Efficacy of desmopressin in the treatment of nocturia: a double-blind placebo-controlled study in men. BJU Int. 2002. 89:855–862.30. Lose G, Lalos O, Freeman RM, van Kerrebroeck P. Nocturia Study Group. Efficacy of desmopressin (Minirin) in the treatment of nocturia: a double-blind placebo-controlled study in women. Am J Obstet Gynecol. 2003. 189:1106–1113.31. van Kerrebroeck P, Rezapour M, Cortesse A, Thuroff J, Riis A, Norgaard JP. Desmopressin in the treatment of nocturia: a double-blind, placebo-controlled study. Eur Urol. 2007. 52:221–229.32. Lose G, Mattiasson A, Walter S, Lalos O, van Kerrebroeck P, Abrams P, et al. Clinical experiences with desmopressin for long-term treatment of nocturia. J Urol. 2004. 172:1021–1025.33. National Institute for Health and Clinical Excellence. NICE clinical guideline 97. Lower urinary tract symptoms: the management of lower urinary tract symptoms in men [Internet]. 2010. cited 2012 Jun 4. London: National Institute for Health and Clinical Excellence;Available from: http://www.nice.org.uk/nicemedia/live/12984/48557/48557.pdf.34. Kaye M. Nocturia: a blinded, randomized, parallel placebo-controlled self-study of the effect of 5 different sedatives and analgesics. Can Urol Assoc J. 2008. 2:604–608.35. Antunes AA, Srougi M, Coelho RF, Leite KR, Freire Gde C. Transurethral resection of the prostate for the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia: how much should be resected? Int Braz J Urol. 2009. 35:683–689.36. Yoshimura K, Ohara H, Ichioka K, Terada N, Matsui Y, Terai A, et al. Nocturia and benign prostatic hyperplasia. Urology. 2003. 61:786–790.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Practical Tip for Management of Nocturia
- The Nocturia in BPH
- Post Treatment Change of Nocturia in Patients with Benign Prostatic Hyperplasia
- Study on Persistent Nocturia after Treatment of Benign Prostatic Hyperplasia and Effectiveness of Desmopressin in Persistent Nocturia with Nocturnal Polyuria
- Nocturia