J Gynecol Oncol.
2012 Oct;23(4):251-256. 10.3802/jgo.2012.23.4.251.
How low is low enough? Evaluation of various risk-assessment models for lymph node metastasis in endometrial cancer: a Korean multicenter study
- Affiliations
-
- 1Center for Uterine Cancer, National Cancer Center, Goyang, Korea. sokbom@gmail.com
- 2Department of Obstetrics and Gynecology, Kyung Hee University School of Medicine, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, Korea University College of Medicine, Seoul, Korea.
- 4Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Obstetrics and Gynecology, Dongsan Medical Center, Keimyung University, Daegu, Korea.
- 6Department of Obstetrics and Gynecology, Chonnam National University Medical School, Gwangju, Korea.
- 7Department of Obstetrics and Gynecology, Gachon University Hospital, Incheon, Korea.
- 8Department of Obstetrics and Gynecology, Inje University Busan Paik Hospital, Busan, Korea.
- KMID: 1810122
- DOI: http://doi.org/10.3802/jgo.2012.23.4.251
Abstract
OBJECTIVE
The aim of this study was to identify a standard for the evaluation of future models for prediction of lymph node metastasis in endometrial cancer through estimation of performance of well-known surgicopathological models.
METHODS
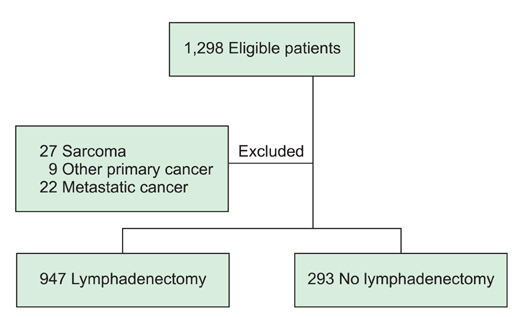
Using the medical records of 947 patients with endometrial cancer who underwent surgical management with lymphadenectomy, we retrospectively assessed the predictive performances of nodal metastasis of currently available models.
RESULTS
We evaluated three models included: 1) a model modified from the Gynecologic Oncology Group (GOG) pilot study; 2) one from the GOG-33 data; and 3) one from Mayo Clinic data. The three models showed similar negative predictive values ranging from 97.1% to 97.4%. Using Bayes' theorem, this can be translated into 2% of negative post-test probability when 10% of prevalence of lymph node metastasis was assumed. In addition, although the negative predictive value was similar among these models, the proportion that was classified as low-risk was significantly different between the studies (56.4%, 44.8%, and 30.5%, respectively; p<0.001).
CONCLUSION
The current study suggests that a false negativity of 2% or less should be a goal for determining clinical usefulness of preoperative or intraoperative prediction models for low-risk of nodal metastasis.
Keyword
MeSH Terms
Figure
Reference
-
1. Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008. 100:1707–1716.2. ASTEC study group. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009. 373:125–136.3. Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri: FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006. 95:Suppl 1. S105–S143.4. Greer BE, Koh WJ, Abu-Rustum N, Bookman MA, Bristow RE, Campos SM, et al. Uterine neoplasms: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009. 7:498–531.5. American College of Obstetricians and Gynecologists. ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: management of endometrial cancer. Obstet Gynecol. 2005. 106:413–425.6. Kitchener HC. To stage or not to stage? That is the question: (with apologies to Shakespeare). Int J Gynecol Cancer. 2010. 20:S55–S56.7. Walsh CS, Karlan BY. Lymphadenectomy's role in early endometrial cancer: prognostic or therapeutic? J Natl Cancer Inst. 2008. 100:1660–1661.8. Uccella S, Podratz KC, Aletti GD, Mariani A. Re: Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2009. 101:897–898.9. Chan JK, Wu H, Cheung MK, Shin JY, Osann K, Kapp DS. The outcomes of 27,063 women with unstaged endometrioid uterine cancer. Gynecol Oncol. 2007. 106:282–288.10. Trimble EL, Kosary C, Park RC. Lymph node sampling and survival in endometrial cancer. Gynecol Oncol. 1998. 71:340–343.11. Lee TS, Kim JW, Kim SH, Seong SJ, Song ES, Kim JH, et al. Surgical practice patterns in endometrial cancer: results of the Korean Gynecologic Oncology Group survey. J Gynecol Oncol. 2009. 20:107–112.12. Boronow RC. Surgical staging of endometrial cancer: evolution, evaluation, and responsible challenge: a personal perspective. Gynecol Oncol. 1997. 66:179–189.13. Boronow RC, Morrow CP, Creasman WT, Disaia PJ, Silverberg SG, Miller A, et al. Surgical staging in endometrial cancer: clinical-pathologic findings of a prospective study. Obstet Gynecol. 1984. 63:825–832.14. Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer: a Gynecologic Oncology Group Study. Cancer. 1987. 60:2035–2041.15. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000. 182:1506–1519.16. Schink JC, Lurain JR, Wallemark CB, Chmiel JS. Tumor size in endometrial cancer: a prognostic factor for lymph node metastasis. Obstet Gynecol. 1987. 70:216–219.17. Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991. 40:55–65.18. Case AS, Rocconi RP, Straughn JM Jr, Conner M, Novak L, Wang W, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006. 108:1375–1379.19. Neubauer NL, Havrilesky LJ, Calingaert B, Bulusu A, Bernardini MQ, Fleming ND, et al. The role of lymphadenectomy in the management of preoperative grade 1 endometrial carcinoma. Gynecol Oncol. 2009. 112:511–516.20. Chan JK, Kapp DS. Role of complete lymphadenectomy in endometrioid uterine cancer. Lancet Oncol. 2007. 8:831–841.21. Mariani A, Dowdy SC, Cliby WA, Gostout BS, Jones MB, Wilson TO, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008. 109:11–18.22. Todo Y, Okamoto K, Hayashi M, Minobe S, Nomura E, Hareyama H, et al. A validation study of a scoring system to estimate the risk of lymph node metastasis for patients with endometrial cancer for tailoring the indication of lymphadenectomy. Gynecol Oncol. 2007. 104:623–628.23. Ballester M, Dubernard G, Lecuru F, Heitz D, Mathevet P, Marret H, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO). Lancet Oncol. 2011. 12:469–476.24. Suh DH, Kim K, Kim JW. Major clinical research advances in gynecologic cancer in 2011. J Gynecol Oncol. 2012. 23:53–64.25. Kang S, Kang WD, Chung HH, Jeong DH, Seo SS, Lee JM, et al. Preoperative identification of a low-risk group for lymph node metastasis in endometrial cancer: a Korean gynecologic oncology group study. J Clin Oncol. 2012. 30:1329–1334.26. Soliman PT, Frumovitz M, Spannuth W, Greer MJ, Sharma S, Schmeler KM, et al. Lymphadenectomy during endometrial cancer staging: practice patterns among gynecologic oncologists. Gynecol Oncol. 2010. 119:291–294.27. Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005. 23:7703–7720.28. Lee KB, Ki KD, Lee JM, Lee JK, Kim JW, Cho CH, et al. The risk of lymph node metastasis based on myometrial invasion and tumor grade in endometrioid uterine cancers: a multicenter, retrospective Korean study. Ann Surg Oncol. 2009. 16:2882–2887.29. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative: Standards for Reporting of Diagnostic Accuracy. Clin Chem. 2003. 49:1–6.30. Chan JK, Kapp DS, Cheung MK, Osann K, Shin JY, Cohn D, et al. The impact of the absolute number and ratio of positive lymph nodes on survival of endometrioid uterine cancer patients. Br J Cancer. 2007. 97:605–611.31. Chi DS, Barakat RR, Palayekar MJ, Levine DA, Sonoda Y, Alektiar K, et al. The incidence of pelvic lymph node metastasis by FIGO staging for patients with adequately surgically staged endometrial adenocarcinoma of endometrioid histology. Int J Gynecol Cancer. 2008. 18:269–273.32. Jaeschke R, Guyatt GH, Sackett DL. The Evidence-Based Medicine Working Group. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? JAMA. 1994. 271:703–707.33. Jaeschke R, Guyatt G, Sackett DL. Evidence-Based Medicine Working Group. Users' guides to the medical literature. III. How to use an article about a diagnostic test. A. Are the results of the study valid? JAMA. 1994. 271:389–391.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparing prediction models for lymph node metastasis risk in endometrial cancer: the winner may not take it all
- A critical assessment on the role of sentinel node mapping in endometrial cancer
- Accuracy Goals in Predicting Preoperative Lymph Node Metastasis for T1 Colorectal Cancer Resected Endoscopically
- Preoperative selection of endometrial cancer patients at low risk for lymph node metastases: useful criteria for enrollment in clinical trials
- A case of minimal uterine serous carcinoma with distant lymph node metastasis without peritoneal dissemination