Ann Surg Treat Res.
2015 Feb;88(2):92-99. 10.4174/astr.2015.88.2.92.
Effect of IL-18 binding protein on hepatic ischemia-reperfusion injury induced by infrarenal aortic occlusion
- Affiliations
-
- 1Department of General Surgery, Faculty of Medicine, Afyon Kocatepe University, Afyon, Turkey. dr.mustafaozsoy@gmail.com
- 2Department of Anatomy, Faculty of Medicine, Afyon Kocatepe University, Afyon, Turkey.
- 3Department of Cardiovascular Surgery, Faculty of Medicine, Afyon Kocatepe University, Afyon, Turkey.
- 4Department of Biochemistry, Faculty of Arts And Sciences, Afyon Kocatepe University, Afyon, Turkey.
- 5Department of Histology and Embryology, Faculty of Medicine, Dumlupinar University, Kutahya, Turkey.
- 6Department of Histology and Embryology, Faculty of Medicine, Afyon Kocatepe University, Afyon, Turkey.
- KMID: 1804126
- DOI: http://doi.org/10.4174/astr.2015.88.2.92
Abstract
- PURPOSE
Severe local and systemic tissue damage called ischemia/reperfusion (IR) injury occurs during the period of reperfusion. Free oxygen radicals and proinflammatory cytokines are responsible for reperfusion injury. IL-18 binding protein (IL-18BP) is a natural inhibitor of IL-18. The balance between IL-18 and IL-18BP has an important role in the inflammatory setting. The present study aimed to investigate whether IL-18BP had a protective role in remote organ hepatic IR injury.
METHODS
Wistar-Albino rats were divided into three groups that contained seven rats. Group I (sham): Laparotomy and infrarenal abdominal aorta (AA) dissection were done but no clamping was done. Group II (I/R): The infrarenal AA was clamped by atraumatic microvascular clamp for 30 minutes and then was exposed to 90 minutes of reperfusion. Group III (IR + IL-18BP): 75 microg/kg of IL-18BP in 0.9% saline (1 mL) was administered 30 minutes before infrarenal AA dissection and clamping; 30 minutes of ischemia was applied and then was exposed to 90 minutes of reperfusion.
RESULTS
Serum AST, ALT, and LDH levels were remarkably higher in IR group and returned to normal levels in treatment group. The proinflammatory cytokine levels had decreased in treatment group, and was statistically significant compared with the IR group. Serum levels of total oxidant status and oxidative stress index decreased and levels of total antioxidant status increased by IL-18BP.
CONCLUSION
This study suggested that IL-18BP has antioxidant, anti-inflammatory and hepatoprotective effects in cases of IR with infrarenal AA induced liver oxidative damage.
Keyword
MeSH Terms
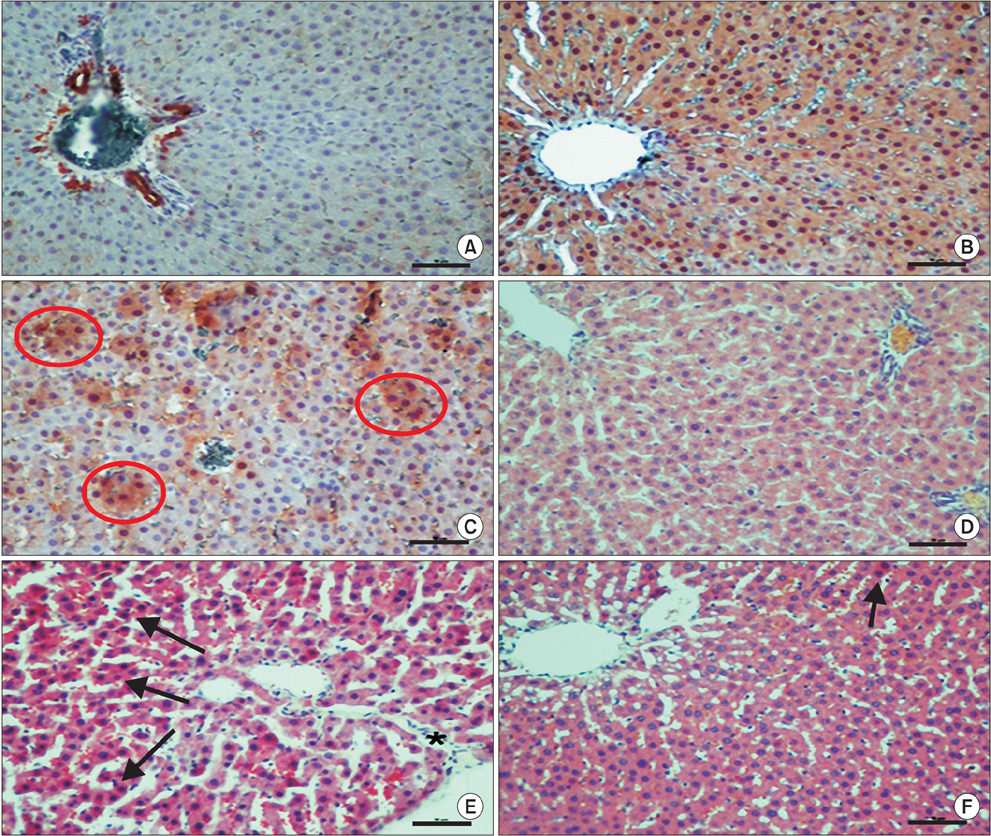
Figure
Reference
-
1. Haimovici H. Arterial embolism with acute massive ischemic myopathy and myoglobinuria: evaluation of a hitherto unreported syndrome with report of two cases. Surgery. 1960; 47:739–747.2. Yang SS, Park KM, Roh YN, Park YJ, Kim DI, Kim YW. Renal and abdominal visceral complications after open aortic surgery requiring supra-renal aortic cross clamping. J Korean Surg Soc. 2012; 83:162–170.3. Clanton TL, Zuo L, Klawitter P. Oxidants and skeletal muscle function: physiologic and pathophysiologic implications. Proc Soc Exp Biol Med. 1999; 222:253–262.4. Boraschi D, Dinarello CA. IL-18 in autoimmunity: review. Eur Cytokine Netw. 2006; 17:224–252.5. Banda NK, Vondracek A, Kraus D, Dinarello CA, Kim SH, Bendele A, et al. Mechanisms of inhibition of collagen-induced arthritis by murine IL-18 binding protein. J Immunol. 2003; 170:2100–2105.6. O'Brien LC, Mezzaroma E, Van Tassell BW, Marchetti C, Carbone S, Abbate A, et al. Interleukin-18 as a therapeutic target in acute myocardial infarction and heart failure. Mol Med. 2014; 20:221–229.7. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005; 38:1103–1111.8. Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004; 37:277–285.9. Erdogan O, Yildiz S, Başaran A, Demirbas A, Yesilkaya A. Effect of intraportal verapamil infusion on hepatic ischemia-reperfusion injury. Pol J Pharmacol. 2001; 53:137–141.10. Ozdulger A, Cinel I, Koksel O, Cinel L, Avlan D, Unlu A, et al. The protective effect of N-acetylcysteine on apoptotic lung injury in cecal ligation and puncture-induced sepsis model. Shock. 2003; 19:366–372.11. Hara M, Yoshida M, Nishijima H, Yokosuka M, Iigo M, Ohtani-Kaneko R, et al. Melatonin, a pineal secretory product with antioxidant properties, protects against cisplatin-induced nephrotoxicity in rats. J Pineal Res. 2001; 30:129–138.12. Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004; 36:1–9.13. Karavelioglu E, Gonul Y, Kokulu S, Hazman O, Bozkurt F, Kocak A, et al. Anti-inflammatory and antiapoptotic effect of interleukine-18 binding protein on the spinal cord ischemia-reperfusion injury. Inflammation. 2014; 37:917–923.14. Tüfek A, Tokgoz O, Aliosmanoglu I, Alabalik U, Evliyaoglu O, Ciftci T, et al. The protective effects of dexmedetomidine on the liver and remote organs against hepatic ischemia reperfusion injury in rats. Int J Surg. 2013; 11:96–100.15. Ono S, Obara H, Takayanagi A, Tanabe M, Kawachi S, Itano O, et al. Suppressive effects of interleukin-18 on liver function in rat liver allografts. J Surg Res. 2012; 176:293–300.16. Dinarello CA. Interleukin-18 and the treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 2004; 30:417–434.17. Dinarello CA. Interleukin-18, a proinflammatory cytokine. Eur Cytokine Netw. 2000; 11:483–486.18. Fantuzzi G, Banda NK, Guthridge C, Vondracek A, Kim SH, Siegmund B, et al. Generation and characterization of mice transgenic for human IL-18-binding protein isoform a. J Leukoc Biol. 2003; 74:889–896.19. Faggioni R, Cattley RC, Guo J, Flores S, Brown H, Qi M, et al. IL-18-binding protein protects against lipopolysaccharide-induced lethality and prevents the development of Fas/Fas ligand-mediated models of liver disease in mice. J Immunol. 2001; 167:5913–5920.20. Venkatachalam K, Prabhu SD, Reddy VS, Boylston WH, Valente AJ, Chandrasekar B. Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. J Biol Chem. 2009; 284:7853–7865.21. Wang Y, Ji M, Chen L, Wu X, Wang L. Breviscapine reduces acute lung injury induced by left heart ischemic reperfusion in rats by inhibiting the expression of ICAM-1 and IL-18. Exp Ther Med. 2013; 6:1322–1326.22. Wang J, Long Q, Zhang W, Chen N. Protective effects of exogenous interleukin 18-binding protein in a rat model of acute renal ischemia-reperfusion injury. Shock. 2012; 37:333–340.23. Wu H, Craft ML, Wang P, Wyburn KR, Chen G, Ma J, et al. IL-18 contributes to renal damage after ischemia-reperfusion. J Am Soc Nephrol. 2008; 19:2331–2341.24. Leslie JA, Meldrum KK. The role of interleukin-18 in renal injury. J Surg Res. 2008; 145:170–175.25. Yang YJ, Shen Y, Chen SH, Ge XR. Role of interleukin 18 in acute lung inflammation induced by gut ischemia reperfusion. World J Gastroenterol. 2005; 11:4524–4529.26. Takeuchi D, Yoshidome H, Kato A, Ito H, Kimura F, Shimizu H, et al. Interleukin 18 causes hepatic ischemia/reperfusion injury by suppressing anti-inflammatory cytokine expression in mice. Hepatology. 2004; 39:699–710.27. Li JM, Eslami MH, Rohrer MJ, Dargon P, Joris I, Hendricks G, et al. Interleukin 18 binding protein (IL18-BP) inhibits neointimal hyperplasia after balloon injury in an atherosclerotic rabbit model. J Vasc Surg. 2008; 47:1048–1057.28. Hwang JM, Cho JS, Kim TH, Lee YI. Ellagic acid protects hepatocytes from damage by inhibiting mitochondrial production of reactive oxygen species. Biomed Pharmacother. 2010; 64:264–270.29. Kim YM, Talanian RV, Li J, Billiar TR. Nitric oxide prevents IL-1beta and IFN-gamma-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1beta-converting enzyme). J Immunol. 1998; 161:4122–4128.30. Camargo CA Jr, Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997; 26:1513–1520.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatic Ischemia-Reperfusion Injury according to Inflow Occlusion in Porcine Liver Surgery
- The Role of HMGB1 and the Effect of Ethyl Pyruvate on Ischemia and Reperfusion Injury of Rat Kidney
- Hepatic ischemia-reperfusion injury with respect to oxidative stress and inflammatory response: a narrative review
- Effect of Superoxide Dismutase and Dimethylthiourea on the Ultrastructure of Hepatocytes in Normothermic Hepatic Ischemia-Reperfusion Injury of Rats
- Spinal Cord Ischemia Related to Infrarenal Aortic Pathology and Surgical Procedure