J Korean Med Sci.
2014 Jan;29(1):2-11. 10.3346/jkms.2014.29.1.2.
Role of Endoplasmic Reticulum Stress in Rheumatoid Arthritis Pathogenesis
- Affiliations
-
- 1Divsion of Rheumatology, Department of Internal Medicine, The Catholic University of Korea School of Medicine, Seoul, Korea. wan725@catholic.ac.kr
- KMID: 1796908
- DOI: http://doi.org/10.3346/jkms.2014.29.1.2
Abstract
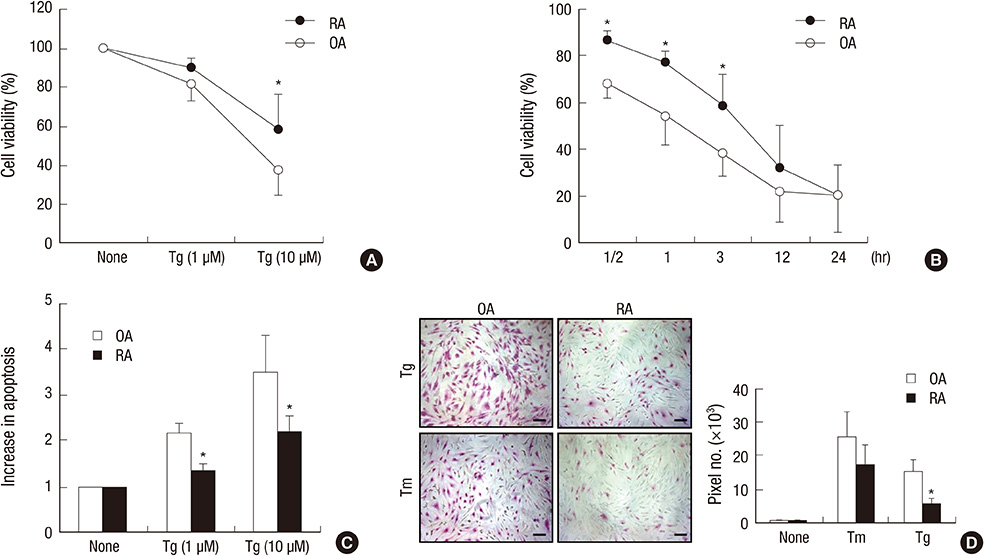
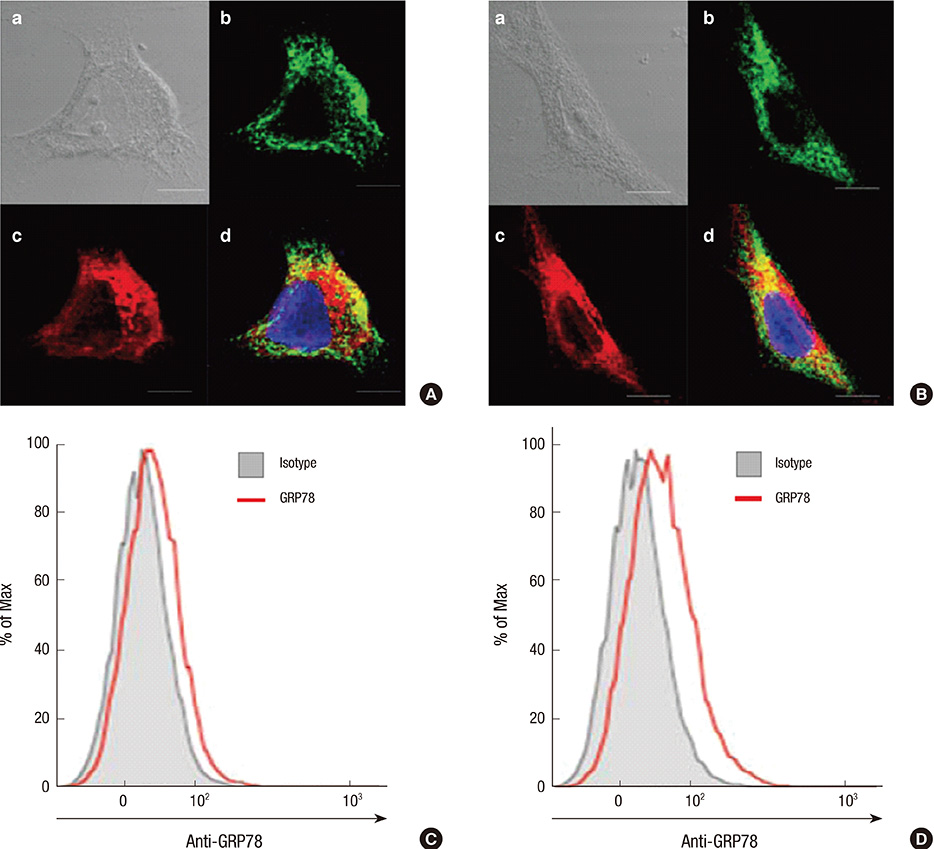
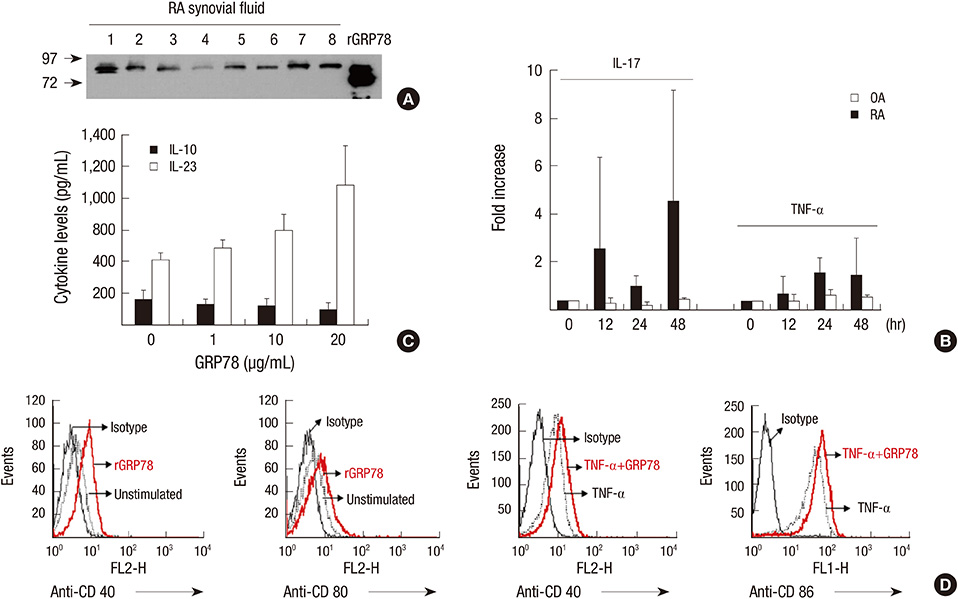
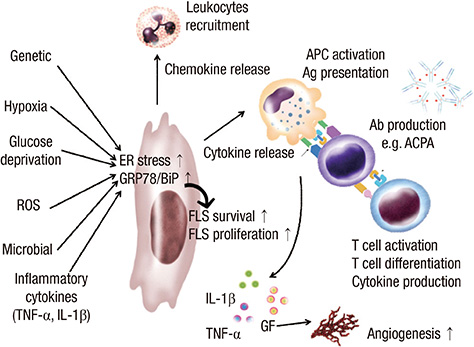
- Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by abnormal proliferation of synoviocytes, leukocyte infiltration, and angiogenesis. The endoplasmic reticulum (ER) is the site of biosynthesis for all secreted and membrane proteins. The accumulation of unfolded proteins in the ER leads to a condition known as ER stress. Failure of the ER's adaptive capacity results in abnormal activation of the unfolded protein response. Recently, we have demonstrated that ER stress-associated gene signatures are highly expressed in RA synovium and synovial cells. Mice with Grp78 haploinsufficiency exhibit the suppression of experimentally induced arthritis, suggesting that the ER chaperone GRP78 is crucial for RA pathogenesis. Moreover, increasing evidence has suggested that GRP78 participates in antibody generation, T cell proliferation, and pro-inflammatory cytokine production, and is therefore one of the potential therapeutic targets for RA. In this review, we discuss the putative, pathophysiological roles of ER stress and GRP78 in RA pathogenesis.
MeSH Terms
-
Animals
Arthritis, Rheumatoid/genetics/*pathology
Autoantibodies/immunology
Cell Proliferation
Cytokines/biosynthesis/immunology
Endoplasmic Reticulum/immunology/pathology
Endoplasmic Reticulum Stress/*immunology
Haploinsufficiency/genetics
Heat-Shock Proteins/*genetics/*immunology
Humans
Lymphocyte Activation
Mice
Neovascularization, Pathologic/genetics
Protein Folding
Synovial Membrane/cytology
T-Lymphocytes/immunology
Unfolded Protein Response/*immunology
Autoantibodies
Cytokines
Heat-Shock Proteins
Figure
Reference
-
1. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011; 365:2205–2219.2. Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis: passive responders or transformed aggressors? Arthritis Rheum. 1996; 39:1781–1790.3. Firestein GS. Starving the synovium: angiogenesis and inflammation in rheumatoid arthritis. J Clin Invest. 1999; 103:3–4.4. Koch AE. Review: angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum. 1998; 41:951–962.5. Jung YO, Kim HA. Recent paradigm shifts in the diagnosis and treatment of rheumatoid arthritis. Korean J Intern Med. 2012; 27:378–387.6. Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012; 181:8–18.7. Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005; 115:2656–2664.8. Hasnain SZ, Lourie R, Das I, Chen AC, McGuckin MA. The interplay between endoplasmic reticulum stress and inflammation. Immunol Cell Biol. 2012; 90:260–270.9. Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008; 454:455–462.10. Yoshida H. ER stress and diseases. FEBS J. 2007; 274:630–658.11. Niederreiter L, Kaser A. Endoplasmic reticulum stress and inflammatory bowel disease. Acta Gastroenterol Belg. 2011; 74:330–333.12. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–529.13. Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005; 74:739–789.14. Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008; 8:663–674.15. Marcinak SJ, Ron D. The unfolded protein response in lung disease. Proc Am Thorac Soc. 2010; 7:356–362.16. Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol. 2013; 12:105–118.17. Kitamura M. Endoplasmic reticulum stress in the kidney. Clin Exp Nephrol. 2008; 12:317–325.18. Nagaraju K, Casciola-Rosen L, Lundberg I, Rawat R, Cutting S, Thapliyal R, Chang J, Dwivedi S, Mitsak M, Chen YW, et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 2005; 52:1824–1835.19. Gordon TP, Bolstad AI, Rischmueller M, Jonsson R, Waterman SA. Autoantibodies in primary Sjögren's syndrome: new insights into mechanisms of autoantibody diversification and disease pathogenesis. Autoimmunity. 2001; 34:123–132.20. Colbert RA, DeLay ML, Klenk EI, Layh-Schmitt G. From HLA-B27 to spondyloarthritis: a journey through the ER. Immunol Rev. 2010; 233:181–202.21. DeLay ML, Turner MJ, Klenk EI, Smith JA, Sowders DP, Colbert RA. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009; 60:2633–2643.22. Turner MJ, Sowders DP, DeLay ML, Mohapatra R, Bai S, Smith JA, Brandewie JR, Taurog JD, Colbert RA. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. J Immunol. 2005; 175:2438–2448.23. Smith JA, Barnes MD, Hong D, DeLay ML, Inman RD, Colbert RA. Gene expression analysis of macrophages derived from ankylosing spondylitis patients reveals interferon-gamma dysregulation. Arthritis Rheum. 2008; 58:1640–1649.24. Dangoria NS, DeLay ML, Kingsbury DJ, Mear JP, Uchanska-Ziegler B, Ziegler A, Colbert RA. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J Biol Chem. 2002; 277:23459–23468.25. Turner MJ, Delay ML, Bai S, Klenk E, Colbert RA. HLA-B27 up-regulation causes accumulation of misfolded heavy chains and correlates with the magnitude of the unfolded protein response in transgenic rats: implications for the pathogenesis of spondylarthritis-like disease. Arthritis Rheum. 2007; 56:215–223.26. Stevens CR, Williams RB, Farrell AJ, Blake DR. Hypoxia and inflammatory synovitis: observations and speculation. Ann Rheum Dis. 1991; 50:124–132.27. Li Z, Li Z. Glucose regulated protein 78: a critical link between tumor microenvironment and cancer hallmarks. Biochim Biophys Acta. 2012; 1826:13–22.28. Verras M, Papandreou I, Lim AL, Denko NC. Tumor hypoxia blocks Wnt processing and secretion through the induction of endoplasmic reticulum stress. Mol Cell Biol. 2008; 28:7212–7224.29. Misra UK, Gonzalez-Gronow M, Gawdi G, Hart JP, Johnson CE, Pizzo SV. The role of Grp 78 in alpha 2-macroglobulin-induced signal transduction: evidence from RNA interference that the low density lipoprotein receptor-related protein is associated with, but not necessary for, GRP 78-mediated signal transduction. J Biol Chem. 2002; 277:42082–42087.30. Misra UK, Deedwania R, Pizzo SV. Activation and cross-talk between Akt, NF-kappaB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J Biol Chem. 2006; 281:13694–13707.31. Misra UK, Wang F, Pizzo SV. Transcription factor TFII-I causes transcriptional upregulation of GRP78 synthesis in prostate cancer cells. J Cell Biochem. 2009; 106:381–389.32. Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003; 278:20915–20924.33. Shu CW, Sun FC, Cho JH, Lin CC, Liu PF, Chen PY, Chang MD, Fu HW, Lai YK. GRP78 and Raf-1 cooperatively confer resistance to endoplasmic reticulum stress-induced apoptosis. J Cell Physiol. 2008; 215:627–635.34. Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001; 22:199–204.35. Qu Z, Garcia CH, O'Rourke LM, Planck SR, Kohli M, Rosenbaum JT. Local proliferation of fibroblast-like synoviocytes contributes to synovial hyperplasia: results of proliferating cell nuclear antigen/cyclin, c-myc, and nucleolar organizer region staining. Arthritis Rheum. 1994; 37:212–220.36. Pap T, Müller-Ladner U, Gay RE, Gay S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2000; 2:361–367.37. Fassbender HG. Histomorphological basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res. 1983; 3:141–155.38. Yoo SA, You S, Yoon HJ, Kim DH, Kim HS, Lee K, Ahn JH, Hwang D, Lee AS, Kim KJ, et al. A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J Exp Med. 2012; 209:871–886.39. Masson-Bessière C, Sebbag M, Durieux JJ, Nogueira L, Vincent C, Girbal-Neuhauser E, Durroux R, Cantagrel A, Serre G. In the rheumatoid pannus, anti-filaggrin autoantibodies are produced by local plasma cells and constitute a higher proportion of IgG than in synovial fluid and serum. Clin Exp Immunol. 2000; 119:544–552.40. Kim HR. Anti-citrullinated protein antibodies in rheumatoid arthritis: a bridge between genetic predisposition and autoimmunity. Korean J Intern Med. 2013; 28:25–28.41. Dörner T, Burmester GR. The role of B cells in rheumatoid arthritis: mechanisms and therapeutic targets. Curr Opin Rheumatol. 2003; 15:246–252.42. Choi SW, Lim MK, Shin DH, Park JJ, Shim SC. Diagnostic performances of anti-cyclic citrullinated peptides antibody and antifilaggrin antibody in Korean patients with rheumatoid arthritis. J Korean Med Sci. 2005; 20:473–478.43. Brewer JW, Hendershot LM. Building an antibody factory: a job for the unfolded protein response. Nat Immunol. 2005; 6:23–29.44. Gass JN, Gunn KE, Sriburi R, Brewer JW. Stressed-out B cells? plasma-cell differentiation and the unfolded protein response. Trends Immunol. 2004; 25:17–24.45. Dong W, Li X, Feng Y, Fan C, Chen Z, Zhu P. The differential expressions of 78-kDa glucose-regulated protein of infiltrating plasma cells in peripheral joints with the histopathological variants of rheumatoid synovitis. Arthritis Res Ther. 2009; 11:R4.46. Bánhegyi G, Baumeister P, Benedetti A, Dong D, Fu Y, Lee AS, Li J, Mao C, Margittai E, Ni M, et al. Endoplasmic reticulum stress. Ann N Y Acad Sci. 2007; 1113:58–71.47. Gass JN, Jiang HY, Wek RC, Brewer JW. The unfolded protein response of B-lymphocytes: PERK-independent development of antibody-secreting cells. Mol Immunol. 2008; 45:1035–1043.48. Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001; 26:504–510.49. Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006; 22:487–508.50. Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005; 35:373–381.51. Corrigall VM, Bodman-Smith MD, Fife MS, Canas B, Myers LK, Wooley P, Soh C, Staines NA, Pappin DJ, Berlo SE, et al. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J Immunol. 2001; 166:1492–1498.52. Shoda H, Fujio K, Shibuya M, Okamura T, Sumitomo S, Okamoto A, Sawada T, Yamamoto K. Detection of autoantibodies to citrullinated BiP in rheumatoid arthritis patients and pro-inflammatory role of citrullinated BiP in collagen-induced arthritis. Arthritis Res Ther. 2011; 13:R191.53. Panayi GS. T-cell-dependent pathways in rheumatoid arthritis. Curr Opin Rheumatol. 1997; 9:236–240.54. Boissier MC, Feng XZ, Carlioz A, Roudier R, Fournier C. Experimental autoimmune arthritis in mice: I. homologous type II collagen is responsible for self-perpetuating chronic polyarthritis. Ann Rheum Dis. 1987; 46:691–700.55. Kakimoto K, Katsuki M, Hirofuji T, Iwata H, Koga T. Isolation of T cell line capable of protecting mice against collagen-induced arthritis. J Immunol. 1988; 140:78–83.56. Park SH, Min DJ, Cho ML, Kim WU, Youn J, Park W, Cho CS, Kim HY. Shift toward T helper 1 cytokines by type II collagen-reactive T cells in patients with rheumatoid arthritis. Arthritis Rheum. 2001; 44:561–569.57. Weiner HL, Zhang ZJ, Khoury SJ, Miller A, Al-Sabbagh A, Brod SA, Lider O, Higgins P, Sobel R, Nussenblatt RB, et al. Antigen-driven peripher al immune tolerance: suppression of organ-specific autoimmune diseases by oral administration of autoantigens. Ann N Y Acad Sci. 1991; 636:227–232.58. Singh VK, Nagaraju K. Experimental autoimmune uveitis: molecular mimicry and oral tolerance. Immunol Res. 1996; 15:323–346.59. Weiner HL, Friedman A, Miller A, Khoury SJ, Al-Sabbagh A, Santos L, Sayegh M, Nussenblatt RB, Trentham DE, Hafler DA. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994; 12:809–837.60. Lee MS. New insight on immune tolerance from transgenic mouse models. J Korean Med Sci. 1996; 11:1–7.61. Corrigall VM, Bodman-Smith MD, Brunst M, Cornell H, Panayi GS. Inhibition of antigen-presenting cell function and stimulation of human peripheral blood mononuclear cells to express an antiinflammatory cytokine profile by the stress protein BiP: relevance to the treatment of inflammatory arthritis. Arthritis Rheum. 2004; 50:1164–1171.62. Luo X, Zuo X, Zhou Y, Zhang B, Shi Y, Liu M, Wang K, McMillian DR, Xiao X. Extracellular heat shock protein 70 inhibits tumour necrosis factor-alpha induced proinflammatory mediator production in fibroblast-like synoviocytes. Arthritis Res Ther. 2008; 10:R41.63. Van Herwijnen MJ, Wieten L, van der Zee R, van Kooten PJ, Wagenaar-Hilbers JP, Hoek A, den Braber I, Anderton SM, Singh M, Meiring HD, et al. Regulatory T cells that recognize a ubiquitous stress-inducible self-antigen are long-lived suppressors of autoimmune arthritis. Proc Natl Acad Sci U S A. 2012; 109:14134–14139.64. Van Anken E, Romijn EP, Maggioni C, Mezghrani A, Sitia R, Braakman I, Heck AJ. Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity. 2003; 18:243–253.65. Lu MC, Lai NS, Yu HC, Huang HB, Hsieh SC, Yu CL. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor alpha production. Arthritis Rheum. 2010; 62:1213–1223.66. Takano S, Ando T, Hiramatsu N, Kanayama A, Maekawa S, Ohnuma Y, Enomoto N, Ogawa H, Paton AW, Paton JC, et al. T cell receptor-mediated signaling induces GRP78 expression in T cells: the implications in maintaining T cell viability. Biochem Biophys Res Commun. 2008; 371:762–766.67. Pino SC, O'Sullivan-Murphy B, Lidstone EA, Thornley TB, Jurczyk A, Urano F, Greiner DL, Mordes JP, Rossini AA, Bortell R. Protein kinase C signaling during T cell activation induces the endoplasmic reticulum stress response. Cell Stress Chaperones. 2008; 13:421–434.68. Chang JS, Ocvirk S, Berger E, Kisling S, Binder U, Skerra A, Lee AS, Haller D. Endoplasmic reticulum stress response promotes cytotoxic phenotype of CD8αβ+ intraepithelial lymphocytes in a mouse model for Crohn's disease-like ileitis. J Immunol. 2012; 189:1510–1520.69. Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006; 124:587–599.70. Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem. 2005; 280:33917–33925.71. Nakajima S, Hiramatsu N, Hayakawa K, Saito Y, Kato H, Huang T, Yao J, Paton AW, Paton JC, Kitamura M. Selective abrogation of BiP/GRP78 blunts activation of NF-κB through the ATF6 branch of the UPR: involvement of C/EBPβ and mTOR-dependent dephosphorylation of Akt. Mol Cell Biol. 2011; 31:1710–1718.72. Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011; 434:181–188.73. Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009; 11:615–628.74. Davidson DJ, Haskell C, Majest S, Kherzai A, Egan DA, Walter KA, Schneider A, Gubbins EF, Solomon L, Chen Z, et al. Kringle 5 of human plasminogen induces apoptosis of endothelial and tumor cells through surface-expressed glucose-regulated protein 78. Cancer Res. 2005; 65:4663–4672.75. Katanasaka Y, Ishii T, Asai T, Naitou H, Maeda N, Koizumi F, Miyagawa S, Ohashi N, Oku N. Cancer antineovascular therapy with liposome drug delivery systems targeted to BiP/GRP78. Int J Cancer. 2010; 127:2685–2698.76. Dong D, Stapleton C, Luo B, Xiong S, Ye W, Zhang Y, Jhaveri N, Zhu G, Ye R, Liu Z, et al. A critical role for GRP78/BiP in the tumor microenvironment for neovascularization during tumor growth and metastasis. Cancer Res. 2011; 71:2848–2857.77. Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjohn J, Talbot UM, Paton JC. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006; 443:548–552.78. Weidle UH, Maisel D, Klostermann S, Schiller C, Weiss EH. Intracellular proteins displayed on the surface of tumor cells as targets for therapeutic intervention with antibody-related agents. Cancer Genomics Proteomics. 2011; 8:49–63.79. Lee SS, Joo YS, Kim WU, Min DJ, Min JK, Park SH, Cho CS, Kim HY. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2001; 19:321–324.80. Lu J, Kasama T, Kobayashi K, Yoda Y, Shiozawa F, Hanyuda M, Negishi M, Ide H, Adachi M. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. J Immunol. 2000; 164:5922–5927.81. Amano T, Yamasaki S, Yagishita N, Tsuchimochi K, Shin H, Kawahara K, Aratani S, Fujita H, Zhang L, Ikeda R, et al. Synoviolin/Hrd1, an E3 ubiquitin ligase, as a novel pathogenic factor for arthropathy. Genes Dev. 2003; 17:2436–2449.82. Kikkert M, Doolman R, Dai M, Avner R, Hassink G, van Voorden S, Thanedar S, Roitelman J, Chau V, Wiertz E. Human HRD1 is an E3 ubiquitin ligase involved in degradation of proteins from the endoplasmic reticulum. J Biol Chem. 2004; 279:3525–3534.83. Hu MC, Gong HY, Lin GH, Hu SY, Chen MH, Huang SJ, Liao CF, Wu JL. XBP-1, a key regulator of unfolded protein response, activates transcription of IGF1 and Akt phosphorylation in zebrafish embryonic cell line. Biochem Biophys Res Commun. 2007; 359:778–783.84. Hampton RY. ER-associated degradation in protein quality control and cellular regulation. Curr Opin Cell Biol. 2002; 14:476–482.85. Yamasaki S, Yagishita N, Tsuchimochi K, Nishioka K, Nakajima T. Rheumatoid arthritis as a hyper-endoplasmic-reticulum-associated degradation disease. Arthritis Res Ther. 2005; 7:181–186.86. Yagishita N, Ohneda K, Amano T, Yamasaki S, Sugiura A, Tsuchimochi K, Shin H, Kawahara K, Ohneda O, Ohta T, et al. Essential role of synoviolin in embryogenesis. J Biol Chem. 2005; 280:7909–7916.