J Korean Med Sci.
2014 Sep;29(9):1217-1225. 10.3346/jkms.2014.29.9.1217.
Benefits of a Continuous Ambulatory Peritoneal Dialysis (CAPD) Technique with One Icodextrin-Containing and Two Biocompatible Glucose-Containing Dialysates for Preservation of Residual Renal Function and Biocompatibility in Incident CAPD Patients
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. kimcmc@catholic.ac.kr
- KMID: 1794599
- DOI: http://doi.org/10.3346/jkms.2014.29.9.1217
Abstract
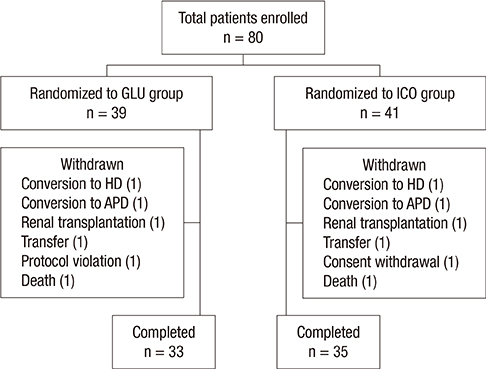
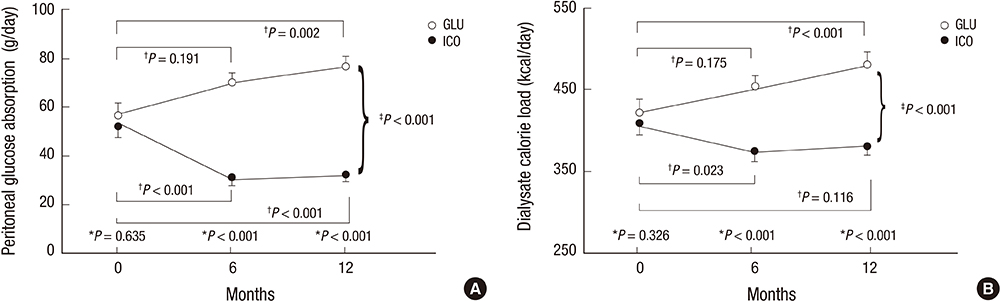
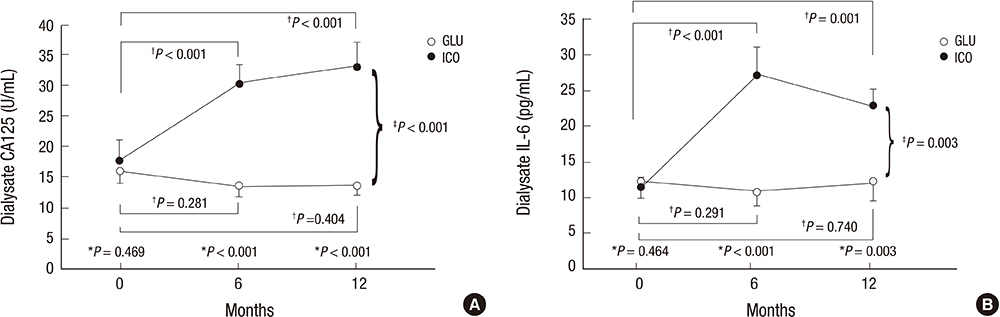
- In a prospective randomized controlled study, the efficacy and safety of a continuous ambulatory peritoneal dialysis (CAPD) technique has been evaluated using one icodextrin-containing and two glucose-containing dialysates a day. Eighty incident CAPD patients were randomized to two groups; GLU group continuously using four glucose-containing dialysates (n=39) and ICO group using one icodextrin-containing and two glucose-containing dialysates (n=41). Variables related to residual renal function (RRF), metabolic and fluid control, dialysis adequacy, and dialysate effluent cancer antigen 125 (CA125) and interleukin 6 (IL-6) levels were measured. The GLU group showed a significant decrease in mean renal urea and creatinine clearance (-Delta1.2+/-2.9 mL/min/1.73 m2, P=0.027) and urine volume (-Delta363.6+/-543.0 mL/day, P=0.001) during 12 months, but the ICO group did not (-Delta0.5+/-2.7 mL/min/1.73 m2, P=0.266; -Delta108.6+/-543.3 mL/day, P=0.246). Peritoneal glucose absorption and dialysate calorie load were significantly lower in the ICO group than the GLU group. The dialysate CA125 and IL-6 levels were significantly higher in the ICO group than the GLU group. Dialysis adequacy, beta2-microglobulin clearance and blood pressure did not differ between the two groups. The CAPD technique using one icodextrin-containing and two glucose-containing dialysates tends to better preserve RRF and is more biocompatible, with similar dialysis adequacy compared to that using four glucose-containing dialysates in incident CAPD patients. [Clincal Trial Registry, ISRCTN23727549]
Keyword
MeSH Terms
-
Adult
Aged
CA-125 Antigen/analysis
Creatinine/urine
Dialysis Solutions/*therapeutic use
Female
Glomerular Filtration Rate
Glucans/*therapeutic use
Glucose/*therapeutic use
Humans
Interleukin-6/analysis
Kidney/physiopathology
Kidney Failure, Chronic/*therapy
Male
Membrane Proteins/analysis
Middle Aged
Peritoneal Dialysis
Peritoneal Dialysis, Continuous Ambulatory
Urea/urine
CA-125 Antigen
Creatinine
Dialysis Solutions
Glucose
Glucans
Interleukin-6
Membrane Proteins
Urea
Figure
Reference
-
1. Bargman JM, Thorpe KE, Churchill DN. CANUSA Peritoneal Dialysis Study Group. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA Study. J Am Soc Nephrol. 2001; 12:2158–2162.2. Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis. 1999; 33:523–534.3. Maiorca R, Brunori G, Zubani R, Cancarini GC, Manili L, Camerini C, Movilli E, Pola A, d'Avolio G, Gelatti U. Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients: a longitudinal study. Nephrol Dial Transplant. 1995; 10:2295–2305.4. Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S. Mexican Nephrology Collaborative Study Group. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002; 13:1307–1320.5. Rocco M, Soucie JM, Pastan S, McClellan WM. Peritoneal dialysis adequacy and risk of death. Kidney Int. 2000; 58:446–457.6. Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT. NECOSAD Study Group. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004; 15:1061–1070.7. Wang AY, Wang M, Woo J, Lam CW, Lui SF, Li PK, Sanderson JE. Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol. 2004; 15:2186–2194.8. Liao CT, Chen YM, Shiao CC, Hu FC, Huang JW, Kao TW, Chuang HF, Hung KY, Wu KD, Tsai TJ. Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol Dial Transplant. 2009; 24:2909–2914.9. Fan SL, Pile T, Punzalan S, Raftery MJ, Yaqoob MM. Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney Int. 2008; 73:200–206.10. Haag-Weber M, Krämer R, Haake R, Islam MS, Prischl F, Haug U, Nabut JL, Deppisch R. behalf of the DIUREST Study Group. Low-GDP fluid (Gambrosol trio) attenuates decline of residual renal function in PD patients: a prospective randomized study. Nephrol Dial Transplant. 2010; 25:2288–2296.11. Kim S, Oh J, Kim S, Chung W, Ahn C, Kim SG, Oh KH. Benefits of biocompatible PD fluid for preservation of residual renal function in incident CAPD patients: a 1-year study. Nephrol Dial Transplant. 2009; 24:2899–2908.12. Szeto CC, Chow KM, Lam CW, Leung CB, Kwan BC, Chung KY, Law MC, Li PK. Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose-degradation products: a 1-year randomized control trial. Nephrol Dial Transplant. 2007; 22:552–559.13. Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, Passlick-Deetjen J. Euro Balance Trial Group. The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int. 2004; 66:408–418.14. Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, Bosselmann HP, Heimbürger O, Simonsen O, Davenport A, et al. Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double-blind randomized controlled trial. J Am Soc Nephrol. 2003; 14:2338–2344.15. Lui SL, Yung S, Yim A, Wong KM, Tong KL, Wong KS, Li CS, Au TC, Lo WK, Ho YW, et al. A combination of biocompatible peritoneal dialysis solutions and residual renal function, peritoneal transport, and inflammation markers: a randomized clinical trial. Am J Kidney Dis. 2012; 60:966–975.16. Takatori Y, Akagi S, Sugiyama H, Inoue J, Kojo S, Morinaga H, Nakao K, Wada J, Makino H. Icodextrin increases technique survival rate in peritoneal dialysis patients with diabetic nephropathy by improving body fluid management: a randomized controlled trial. Clin J Am Soc Nephrol. 2011; 6:1337–1344.17. Dombros N, Dratwa M, Feriani M, Gokal R, Heimbürger O, Krediet R, Plum J, Rodrigues A, Selgas R, Struijk D, et al. European best practice guidelines for peritoneal dialysis: 7 adequacy of peritoneal dialysis. Nephrol Dial Transplant. 2005; 20:ix24–ix27.18. National Kidney Foundation. KDOQI Clinical practice guidelines for peritoneal dialysis adequecy: guideline 2. peritoneal dialysis solute clearance targets and measurements. Am J Kidney Dis. 2006; 49:S103–S116.19. De Vecchi AF, Scalamogna A, Finazzi S, Colucci P, Ponticelli C. Preliminary evaluation of incremental peritoneal dialysis in 25 patients. Perit Dial Int. 2000; 20:412–417.20. Viglino G, Neri L, Barbieri S. Incremental peritoneal dialysis: effects on the choice of dialysis modality, residual renal function and adequacy. Kidney Int Suppl. 2008; (108):S52–S55.21. Bergström J, Heimbürger O, Lindholm B. Calculation of the protein equivalent of total nitrogen appearance from urea appearance: which formulas should be used. Perit Dial Int. 1998; 18:467–473.22. Gokal R, Moberly J, Lindholm B, Mujais S. Metabolic and laboratory effects of icodextrin. Kidney Int Suppl. 2002; (81):S62–S71.23. Bouchi R, Babazono T, Inoue A, Tanaka M, Tanaka N, Hase M, Ishii A, Iwamoto Y. Icodextrin increases natriuretic peptides in diabetic patients undergoing CAPD. Perit Dial Int. 2006; 26:604–607.24. Davies SJ, Garcia Lopez E, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, Bosselmann HP, Heimburger O, Simonsen O, et al. Longitudinal relationships between fluid status, inflammation, urine volume and plasma metabolites of icodextrin in patients randomized to glucose or icodextrin for the long exchange. Nephrol Dial Transplant. 2008; 23:2982–2988.25. Kendrick J, Teitelbaum I. Strategies for improving long-term survival in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2010; 5:1123–1131.26. Finkelstein F, Healy H, Abu-Alfa A, Ahmad S, Brown F, Gehr T, Nash K, Sorkin M, Mujais S. Superiority of icodextrin compared with 4.25% dextrose for peritoneal ultrafiltration. J Am Soc Nephrol. 2005; 16:546–554.27. Konings CJ, Kooman JP, Schonck M, Gladziwa U, Wirtz J, van den Wall Bake AW, Gerlag PG, Hoorntje SJ, Wolters J, van der Sande FM, et al. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: a randomized study. Kidney Int. 2003; 63:1556–1563.28. Wolfson M, Piraino B, Hamburger RJ, Morton AR. Icodextrin Study Group. A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J Kidney Dis. 2002; 40:1055–1065.29. Woodrow G, Oldroyd B, Stables G, Gibson J, Turney JH, Brownjohn AM. Effects of icodextrin in automated peritoneal dialysis on blood pressure and bioelectrical impedance analysis. Nephrol Dial Transplant. 2000; 15:862–866.30. García-López E, Lindholm B, Davies S. An update on peritoneal dialysis solutions. Nat Rev Nephrol. 2012; 8:224–233.31. Oda H, Keane WF. Lipid abnormalities in end stage renal disease. Nephrol Dial Transplant. 1998; 13:45–49.32. Fernström A, Hylander B, Moritz A, Jacobsson H, Rössner S. Increase of intra-abdominal fat in patients treated with continuous ambulatory peritoneal dialysis. Perit Dial Int. 1998; 18:166–171.33. Mistry CD, Gokal R, Peers E. A randomized multicenter clinical trial comparing isosmolar icodextrin with hyperosmolar glucose solutions in CAPD: MIDAS Study Group: Multicenter Investigation of Icodextrin in Ambulatory Peritoneal Dialysis. Kidney Int. 1994; 46:496–503.34. Visser CE, Brouwer-Steenbergen JJ, Betjes MG, Koomen GC, Beelen RH, Krediet RT. Cancer antigen 125: a bulk marker for the mesothelial mass in stable peritoneal dialysis patients. Nephrol Dial Transplant. 1995; 10:64–69.35. Schwenger V, Morath C, Salava A, Amann K, Seregin Y, Deppisch R, Ritz E, Bierhaus A, Nawroth PP, Zeier M. Damage to the peritoneal membrane by glucose degradation products is mediated by the receptor for advanced glycation end-products. J Am Soc Nephrol. 2006; 17:199–207.36. Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002; 4:S233–S242.37. Martikainen T, Ekstrand A, Honkanen E, Teppo AM, Grönhagen-Riska C. Do interleukin-6, hyaluronan, soluble intercellular adhesion molecule-1 and cancer antigen 125 in dialysate predict changes in peritoneal function? a 1-year follow-up study. Scand J Urol Nephrol. 2005; 39:410–416.38. Witowski J, Topley N, Jörres A, Liberek T, Coles GA, Williams JD. Effect of lactate-buffered peritoneal dialysis fluids on human peritoneal mesothelial cell interleukin-6 and prostaglandin synthesis. Kidney Int. 1995; 47:282–293.39. Bruschi M, Candiano G, Santucci L, Petretto A, Mangraviti S, Canepa A, Perri K, Ghiggeri GM, Verrina E. Proteome profile of peritoneal effluents in children on glucose- or icodextrin-based peritoneal dialysis. Nephrol Dial Transplant. 2011; 26:308–316.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathogenesis and Treatment of Dyskalemia in Maintenance Hemodialysis and CAPD
- Comparison of Icodextrin and 2.5% Glucose in Potassium Metabolism by Acute K+oad via Dialysate in Continuous Ambulatory Peritoneal Dialysis Patients
- Spontaneous Renal Rupture with Hemoperitoneum in a Patient on Continuous Ambulatory Peritoneal Dialysis
- Chylous Ascites in a Patient Undergoing Continuous Ambulatory Peritoneal Dialysis
- Severe hypoglycemia due to falsely elevated capillary blood glucose levels in a peritoneal dialysis patient using icodextrin: A case report