Electrolyte Blood Press.
2009 Jun;7(1):25-30. 10.5049/EBP.2009.7.1.25.
Comparison of Icodextrin and 2.5% Glucose in Potassium Metabolism by Acute K+oad via Dialysate in Continuous Ambulatory Peritoneal Dialysis Patients
- Affiliations
-
- 1Department of Internal Medicine, Hanyang University Guri Hospital, Guri, Korea. kimhj@hanyang.ac.kr
- KMID: 1464786
- DOI: http://doi.org/10.5049/EBP.2009.7.1.25
Abstract
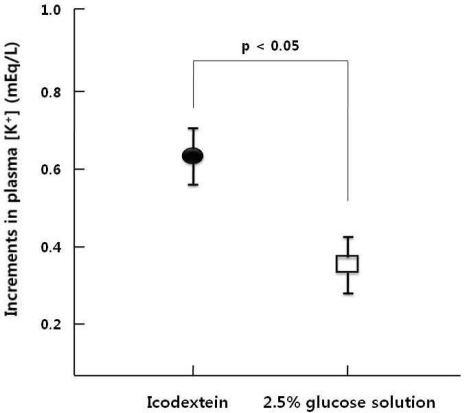
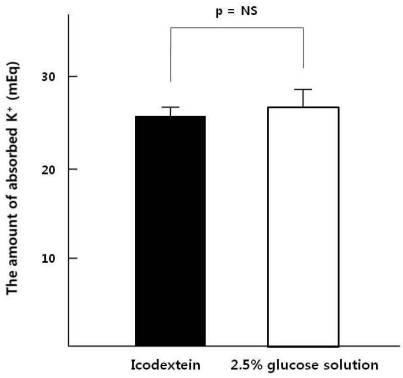
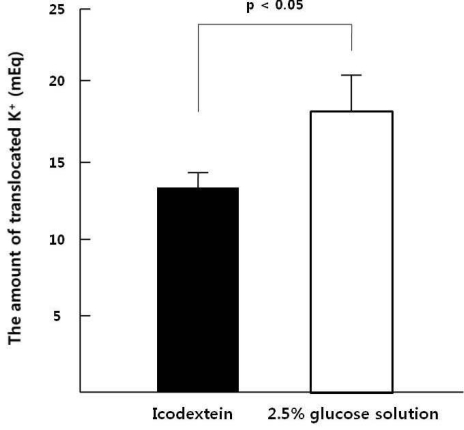
- This study aimed to compare the increment in plasma potassium concentration ([K+]) as well as the role of internal K+ balance for its changes following acute K+ supplementation between conventional 2.5% glucose (GD) and non-glucose containing dialysate (icodextrin, ID) in continuous ambulatory peritoneal dialysis (CAPD) patients. A total of 9 stable CAPD patients (5 men and 4 women; age, 56+/-13 years; 7 type-2 diabetics and 2 non-diabetics) on daily 4 exchanges of 2 L of glucose dialysate underwent the 6-hr dwell on fasting in the morning with 2 L of 2.5% glucose mixed with 20 mEq/L of KCl, and then the same regimen was repeated with icodextrin after 1-wk interval. The degree of intraperitoneal absorption was comparable, 65+/-2% in GD and 68+/-2% in ID, respectively (p=NS). However, despite the similar plasma K+ levels at the baseline of both regimens, its increment was significantly less in GD than ID, which was accompanied by more marked increase in the calculated intracellular K+ redistribution (68+/-3% vs. 52+/-3%, p<0.05). The basal levels of insulin were similar between the GD and ID groups. However, the change, checked up after 2 hours' dwell, from the basal insulin levels was much lower on ID. ID with a lesser degree of transcelluar K+ shift by the decreased secretion of insulin is more effective than the conventional glucose solution for acute K+ repletion via dialysate during CAPD. Furthermore, these results suggested that the role of insulin for the internal K+ balance was intact even in type-2 diabetic patients on CAPD
Keyword
MeSH Terms
Figure
Reference
-
1. Oreopoulos DG, Khanna R, Williams P, Vas SI. Continuous ambulatory peritoneal dialysis-1981. Nephron. 1982; 30:293–303. PMID: 7110460.2. Rostand SG. Profound hypokalemia in continuous ambulatory peritoneal dialysis. Arch Intern Med. 1983; 143:377–378. PMID: 6824407.
Article3. Spital A, Sterns RH. Potassium supplementation via the dialysate in continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1985; 6:173–176. PMID: 3898826.
Article4. Khan AN, Bernardini J, Johnston JR, Piraino B. Hypokalemia in peritoneal dialysis patients. Perit Dial Int. 1996; 16:652. PMID: 8981546.
Article5. Szeto CC, Chow KM, Kwan BC, Leung CB, Chung KY, Law MC, et al. Hypokalemia in Chinese peritoneal dialysis patients: prevalence and prognostic implication. Am J Kidney Dis. 2005; 46:128–135. PMID: 15983966.
Article6. McIntyre CW. Update on peritoneal dialysis solutions. Kidney Int. 2007; 71:486–490. PMID: 17299524.
Article7. Finkelstein F, Healy H, Abu-Alfa A, Ahmad S, Brown F, Gehr T, et al. Superiority of icodextrin compared with 4.25% dextrose for peritoneal ultrafiltration. J Am Soc Nephrol. 2005; 16:546–554. PMID: 15625070.
Article8. Wiggins KJ, Rumpsfeld M, Blizzard S, Johnson DW. Predictors of a favourable response to icodextrin in peritoneal dialysis patients with ultrafiltration failure. Nephrology (Carlton). 2005; 10:33–36. PMID: 15705179.
Article9. Amici G, Orrasch M, Da Rin G, Bocci C. Hyperinsulinism reduction associated with icodextrin treatment in continuous ambulatory peritoneal dialysis patients. Adv Perit Dial. 2001; 17:80–83. PMID: 11510303.10. Tzamaloukas AH, Avasthi PS. Temporal profile of serum potassium concentration in nondiabetic and diabetic outpatients on chronic dialysis. Am J Nephrol. 1987; 7:101–109. PMID: 3605230.
Article11. Brown ST, Ahearn DJ, Nolph KD. Potassium removal with peritoneal dialysis. Kidney Int. 1973; 4:67–69. PMID: 4723995.
Article12. Bergstrom J, Alvestrand A, Furst P, Hultman E, Widstam-Attorps U. Muscle intracellular electrolytes in patients with chronic uremia. Kidney Int Suppl. 1983; 16:S153–S160. PMID: 6588246.13. Lindholm B, Alvestrand A, Hultman E, Bergstrom J. Muscle water and electrolytes in patients undergoing continuous ambulatory peritoneal dialysis. Acta Med Scand. 1986; 219:323–330. PMID: 3706006.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Severe hypoglycemia due to falsely elevated capillary blood glucose levels in a peritoneal dialysis patient using icodextrin: A case report
- Icodextrin Improves the Serum Potassium Profile with the Enhancement of Nutritional Status in Continuous Ambulatory Peritoneal Dialysis Patients
- Pathogenesis and Treatment of Dyskalemia in Maintenance Hemodialysis and CAPD
- A case of hydrothorax caused by peritoneal dialysate leakage diagnosed using the reaction between icodextrin and povidone-iodine
- Blood glucose analysis in patients on continuous ambulatory peritoneal dialysis with Icodextrin