Yonsei Med J.
2013 Sep;54(5):1127-1136. 10.3349/ymj.2013.54.5.1127.
Reduced Food Intake is the Major Contributor to the Protective Effect of Rimonabant on Islet in Established Obesity-Associated Type 2 Diabetes
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jaehyeon@skku.edu
- 2Samsung Biomedical Research Institute, Seoul, Korea.
- 3Diabetes Research Institute, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Food and Nutrition, Chonnam National University, Gwangju, Korea.
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. cyber.park@samsung.com
- KMID: 1793159
- DOI: http://doi.org/10.3349/ymj.2013.54.5.1127
Abstract
- PURPOSE
Although the presence of cannabinoid type 1 (CB1) receptor in islets has been reported, the major contributor to the protective effect of rimonabant on islet morphology is unknown. We determined whether the protective effect of rimonabant on pancreatic islet morphology is valid in established diabetes and also whether any effect was independent of decreased food intake.
MATERIALS AND METHODS
After diabetes was confirmed, Otsuka Long-Evans Tokushima Fatty rats, aged 32 weeks, were treated with rimonabant (30 mg/kg/d, rimonabant group) for 6 weeks. Metabolic profiles and islet morphology of rats treated with rimonabant were compared with those of controls without treatment (control group), a pair-fed control group, and rats treated with rosiglitazone (4 mg/kg/d, rosiglitazone group).
RESULTS
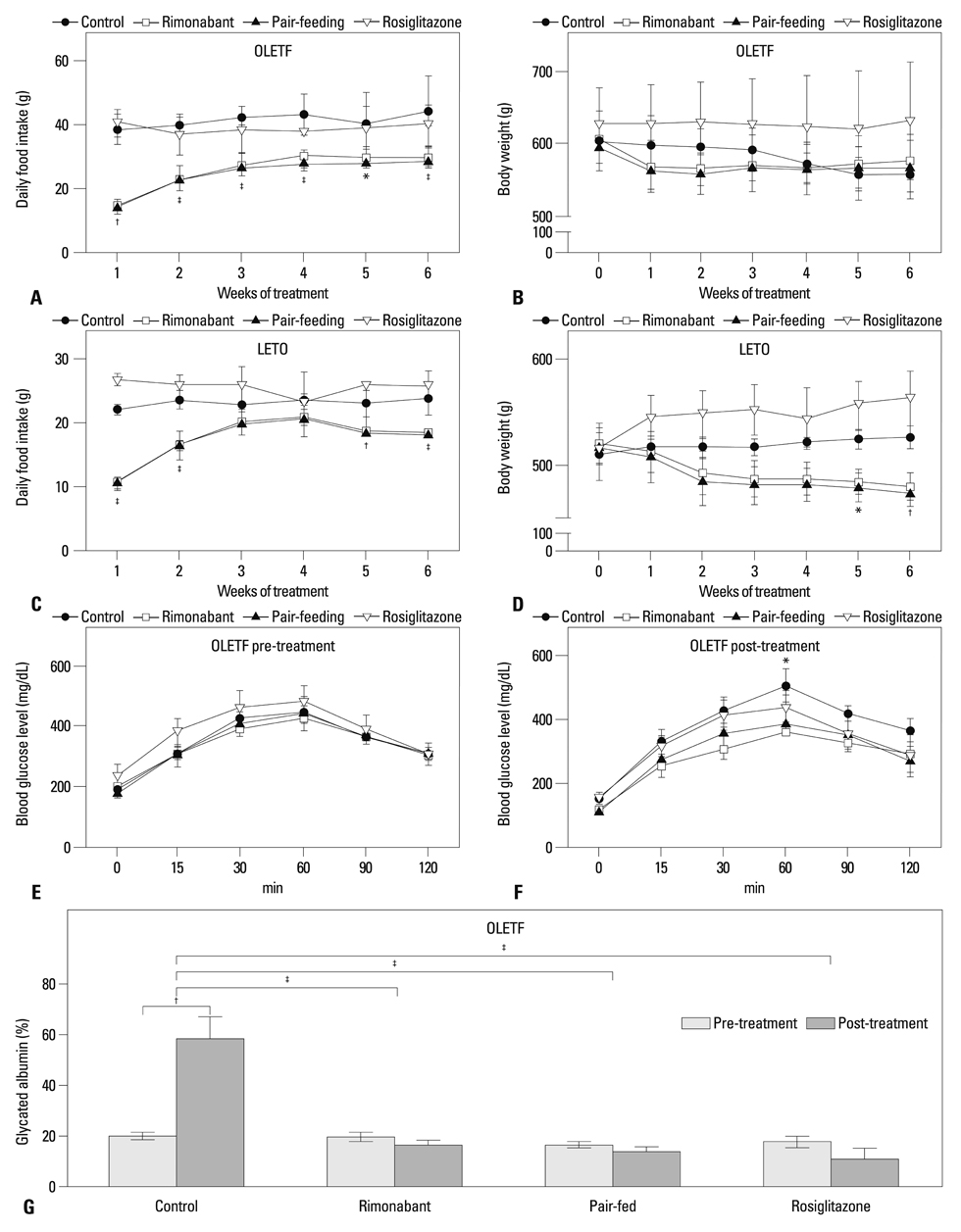
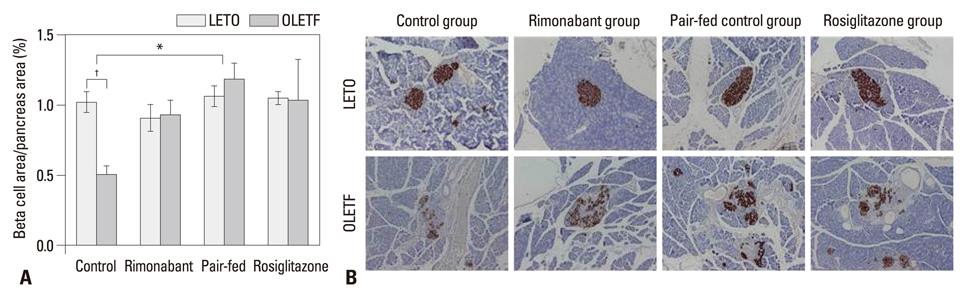
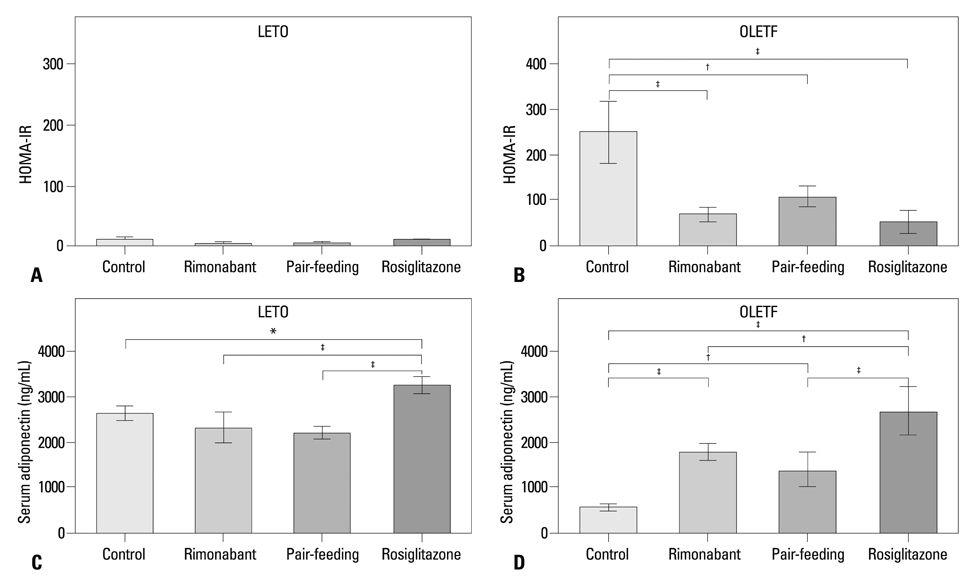
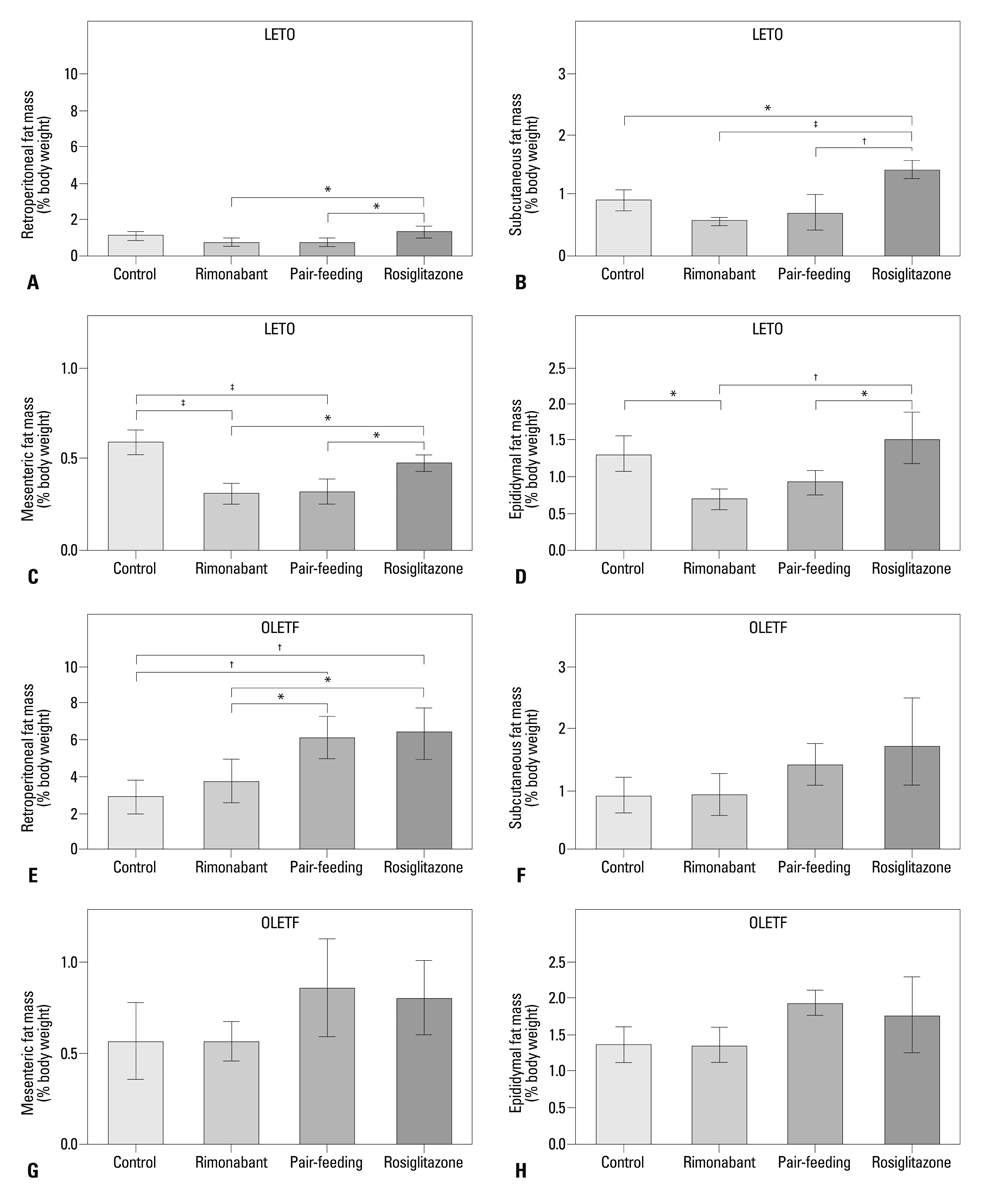
Compared to the control group, rats treated with rimonabant exhibited reduced glycated albumin levels (p<0.001), islet fibrosis (p<0.01), and improved glucose tolerance (p<0.05), with no differences from the pair-fed control group. The retroperitoneal adipose tissue mass was lower in the rimonabant group than those of the pair-fed control and rosiglitazone groups (p<0.05). Rimonabant, pair-fed control, and rosiglitazone groups showed decreased insulin resistance and increased adiponectin, with no differences between the rimonabant and pair-fed control groups.
CONCLUSION
Rimonabant had a protective effect on islet morphology in vivo even in established diabetes. However, the protective effect was also reproduced by pair-feeding. Thus, the results of this study did not support the significance of islet CB1 receptors in islet protection with rimonabant in established obesity-associated type 2 diabetes.
Keyword
MeSH Terms
-
Adiponectin/metabolism
Adiposity/drug effects
Animals
Cell Proliferation/drug effects
Diabetes Mellitus, Type 2/diet therapy/*drug therapy
Eating/*drug effects
Glucose Intolerance/diet therapy/*drug therapy
Insulin Resistance
Insulin-Secreting Cells/*drug effects/pathology
Male
Piperidines/adverse effects/*therapeutic use
Pyrazoles/adverse effects/*therapeutic use
Rats
Rats, Inbred OLETF
Receptor, Cannabinoid, CB1/physiology
Thiazolidinediones/*therapeutic use
Adiponectin
Piperidines
Pyrazoles
Receptor, Cannabinoid, CB1
Thiazolidinediones
Figure
Reference
-
1. Koh EH, Kim MS, Park JY, Kim HS, Youn JY, Park HS, et al. Peroxisome proliferator-activated receptor (PPAR)-alpha activation prevents diabetes in OLETF rats: comparison with PPAR-gamma activation. Diabetes. 2003; 52:2331–2337.
Article2. Duvivier VF, Delafoy-Plasse L, Delion V, Lechevalier P, Le Bail JC, Guillot E, et al. Beneficial effect of a chronic treatment with rimonabant on pancreatic function and beta-cell morphology in Zucker Fatty rats. Eur J Pharmacol. 2009; 616:314–320.
Article3. Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T, et al. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001; 50:1021–1029.4. Janiak P, Poirier B, Bidouard JP, Cadrouvele C, Pierre F, Gouraud L, et al. Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int. 2007; 72:1345–1357.
Article5. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356:2457–2471.
Article6. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007; 298:1189–1195.
Article7. Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP Jr, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011; 34:916–922.
Article8. Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011; 34:1369–1371.
Article9. Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007; 370:1706–1713.
Article10. Bermudez-Silva FJ, Viveros MP, McPartland JM, Rodriguez de Fonseca F. The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacol Biochem Behav. 2010; 95:375–382.
Article11. Pavon FJ, Bilbao A, Hernández-Folgado L, Cippitelli A, Jagerovic N, Abellán G, et al. Antiobesity effects of the novel in vivo neutral cannabinoid receptor antagonist 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1H-1,2,4-triazole--LH 21. Neuropharmacology. 2006; 51:358–366.
Article12. Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007; 91:383–388.
Article13. Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010; 120:2953–2966.
Article14. Nakata M, Yada T. Cannabinoids inhibit insulin secretion and cytosolic Ca2+ oscillation in islet beta-cells via CB1 receptors. Regul Pept. 2008; 145:49–53.
Article15. Vilches-Flores A, Delgado-Buenrostro NL, Navarrete-Vázquez G, Villalobos-Molina R. CB1 cannabinoid receptor expression is regulated by glucose and feeding in rat pancreatic islets. Regul Pept. 2010; 163:81–87.
Article16. Getty-Kaushik L, Richard AM, Deeney JT, Krawczyk S, Shirihai O, Corkey BE. The CB1 antagonist rimonabant decreases insulin hypersecretion in rat pancreatic islets. Obesity (Silver Spring). 2009; 17:1856–1860.
Article17. Li C, Bowe JE, Huang GC, Amiel SA, Jones PM, Persaud SJ. Cannabinoid receptor agonists and antagonists stimulate insulin secretion from isolated human islets of Langerhans. Diabetes Obes Metab. 2011; 13:903–910.
Article18. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992; 41:1422–1428.
Article19. Ishida K, Mizuno A, Min Z, Sano T, Shima K. Which is the primary etiologic event in Otsuka Long-Evans Tokushima Fatty rats, a model of spontaneous non-insulin-dependent diabetes mellitus, insulin resistance, or impaired insulin secretion? Metabolism. 1995; 44:940–945.
Article20. Watanabe T, Kubota N, Ohsugi M, Kubota T, Takamoto I, Iwabu M, et al. Rimonabant ameliorates insulin resistance via both adiponectin-dependent and adiponectin-independent pathways. J Biol Chem. 2009; 284:1803–1812.
Article21. Yang SJ, Choi JM, Chae SW, Kim WJ, Park SE, Rhee EJ, et al. Activation of peroxisome proliferator-activated receptor gamma by rosiglitazone increases sirt6 expression and ameliorates hepatic steatosis in rats. PLoS One. 2011; 6:e17057.
Article22. Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta. 2002; 324:61–71.
Article23. Kouzuma T, Uemastu Y, Usami T, Imamura S. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta. 2004; 346:135–143.
Article24. Li C, Bowe JE, Jones PM, Persaud SJ. Expression and function of cannabinoid receptors in mouse islets. Islets. 2010; 2:293–302.
Article25. Bermúdez-Silva FJ, Suárez J, Baixeras E, Cobo N, Bautista D, Cuesta-Muñoz AL, et al. Presence of functional cannabinoid receptors in human endocrine pancreas. Diabetologia. 2008; 51:476–487.
Article26. Vettor R, Pagano C. The role of the endocannabinoid system in lipogenesis and fatty acid metabolism. Best Pract Res Clin Endocrinol Metab. 2009; 23:51–63.
Article27. Mølhøj S, Hansen HS, Schweiger M, Zimmermann R, Johansen T, Malmlöf K. Effect of the cannabinoid receptor-1 antagonist rimonabant on lipolysis in rats. Eur J Pharmacol. 2010; 646:38–45.
Article28. Moran TH, Katz LF, Plata-Salaman CR, Schwartz GJ. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol. 1998; 274(3 Pt 2):R618–R625.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Macronutrient Distributions in Nutrition Recommendation for Type 2 Diabetes Management
- Amylin Analogue in the Treatment of Obesity
- Antiobesity Pharmacotherapy for Patients with Type 2 Diabetes: Focus on Long-Term Management
- The Efficacy and Safety of Non-Nutritive Sweeteners
- New and emerging drugs in type 2 diabetes