J Korean Med Sci.
2013 Aug;28(8):1161-1168. 10.3346/jkms.2013.28.8.1161.
An Integrative Model of the Cardiovascular System Coupling Heart Cellular Mechanics with Arterial Network Hemodynamics
- Affiliations
-
- 1Department of Mechanical and Biomedical Engineering, Kangwon National University, Chuncheon, Korea. ebshim@kangwon.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, Seoul National University College of Medicine and SMG-SNU Boramae Hospital, Seoul, Korea.
- 3Department of Information and Communications Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea.
- KMID: 1793020
- DOI: http://doi.org/10.3346/jkms.2013.28.8.1161
Abstract
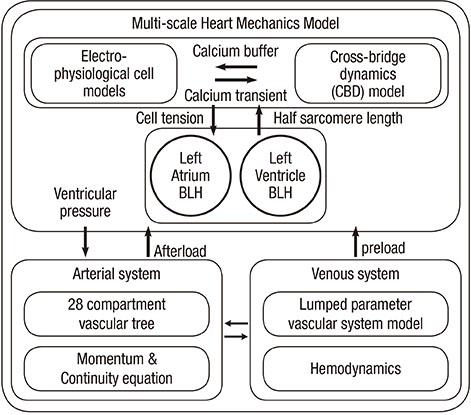
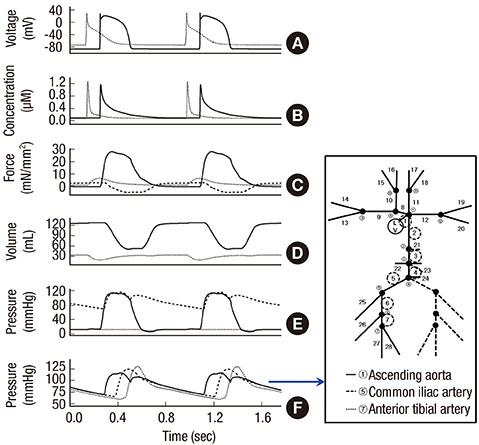
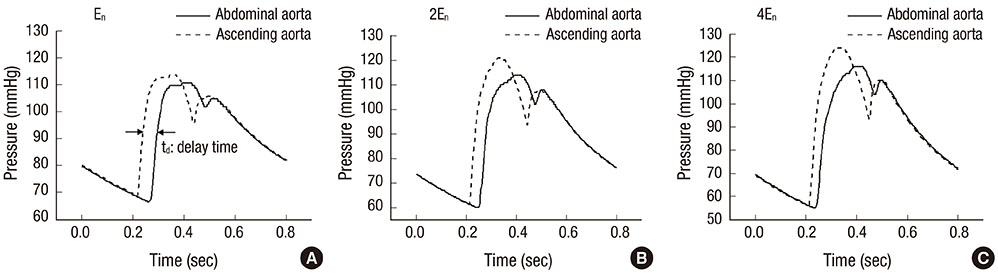
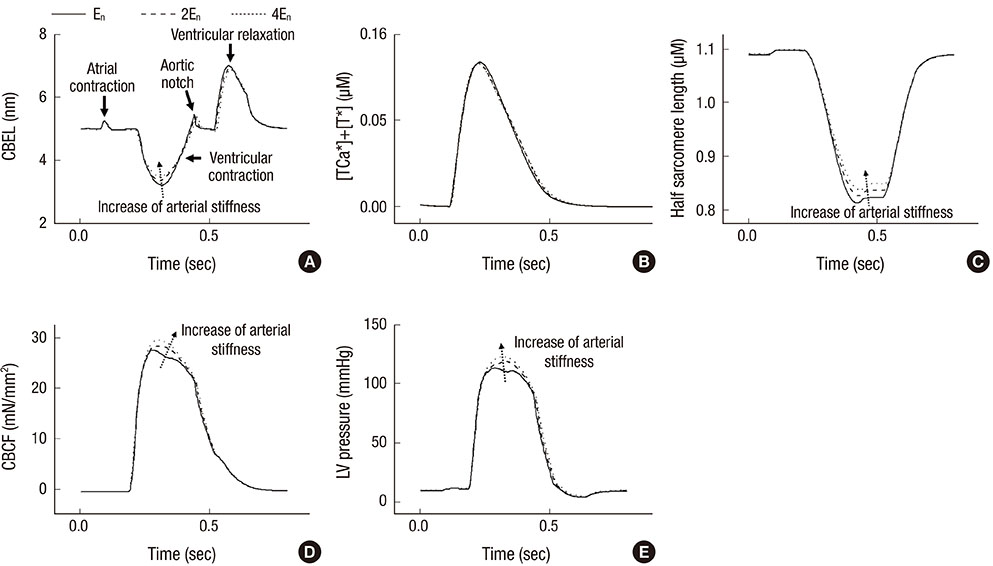
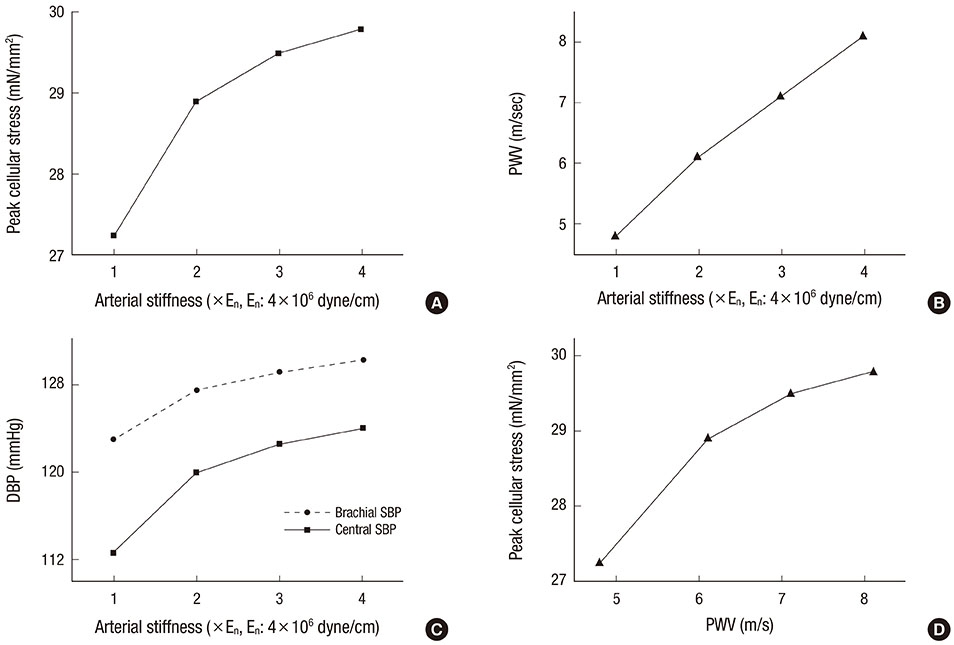
- The current study proposes a model of the cardiovascular system that couples heart cell mechanics with arterial hemodynamics to examine the physiological role of arterial blood pressure (BP) in left ventricular hypertrophy (LVH). We developed a comprehensive multiphysics and multiscale cardiovascular model of the cardiovascular system that simulates physiological events, from membrane excitation and the contraction of a cardiac cell to heart mechanics and arterial blood hemodynamics. Using this model, we delineated the relationship between arterial BP or pulse wave velocity and LVH. Computed results were compared with existing clinical and experimental observations. To investigate the relationship between arterial hemodynamics and LVH, we performed a parametric study based on arterial wall stiffness, which was obtained in the model. Peak cellular stress of the left ventricle and systolic blood pressure (SBP) in the brachial and central arteries also increased; however, further increases were limited for higher arterial stiffness values. Interestingly, when we doubled the value of arterial stiffness from the baseline value, the percentage increase of SBP in the central artery was about 6.7% whereas that of the brachial artery was about 3.4%. It is suggested that SBP in the central artery is more critical for predicting LVH as compared with other blood pressure measurements.
Keyword
MeSH Terms
Figure
Reference
-
1. Abhayaratna WP, Barnes ME, O'Rourke MF, Gersh BJ, Seward JB, Miyasaka Y, Bailey KR, Tsang TS. Relation of arterial stiffness to left ventricular diastolic function and cardiovascular risk prediction in patients > or =65 years of age. Am J Cardiol. 2006; 98:1387–1392.2. Urbina EM, Dolan LM, McCoy CE, Khoury PR, Daniels SR, Kimball TR. Relationship between elevated arterial stiffness and increased left ventricular mass in adolescents and young adults. J Pediatr. 2011; 158:715–721.3. Kerckhoffs RC, Neal ML, Gu Q, Bassingthwaighte JB, Omens JH, McCulloch AD. Coupling of a 3D finite element model of cardiac ventricular mechanics to lumped systems models of the systemic and pulmonic circulation. Ann Biomed Eng. 2007; 35:1–18.4. Lim KM, Lee JS, Song JH, Youn CH, Choi JS, Shim EB. Theoretical estimation of cannulation methods for left ventricular assist device support as a bridge to recovery. J Korean Med Sci. 2011; 26:1591–1598.5. Trayanova NA, Rice JJ. Cardiac electromechanical models: from cell to organ. Front Physiol. 2011; 2:43.6. Campbell SG, McCulloch AD. Multi-scale computational models of familial hypertrophic cardiomyopathy: genotype to phenotype. J R Soc Interface. 2011; 8:1550–1561.7. Shim EB, Sah JY, Youn CH. Mathematical modeling of cardiovascular system dynamics using a lumped parameter method. Jpn J Physiol. 2004; 54:545–553.8. Shim EB, Leem CH, Abe Y, Noma A. A new multi-scale simulation model of the circulation: from cells to system. Philos Trans A Math Phys Eng Sci. 2006; 364:1483–1500.9. Shim EB, Amano A, Takahata T, Shimayoshi T, Noma A. The cross-bridge dynamics during ventricular contraction predicted by coupling the cardiac cell model with a circulation model. J Physiol Sci. 2007; 57:275–285.10. Shim EB, Jun HM, Leem CH, Matusuoka S, Noma A. A new integrated method for analyzing heart mechanics using a cell-hemodynamics-autonomic nerve control coupled model of the cardiovascular system. Prog Biophys Mol Biol. 2008; 96:44–59.11. Ozawa ET, Bottom KE, Xiao X, Kamm RD. Numerical simulation of enhanced external counterpulsation. Ann Biomed Eng. 2001; 29:284–297.12. Stettler JC, Niederer P, Anliker M. Theoretical analysis of arterial hemodynamics including the influence of bifurcations: part I: mathematical models and prediction of normal pulse patterns. Ann Biomed Eng. 1981; 9:145–164.13. Avolio AP. Multi-branched model of the human arterial system. Med Biol Eng Comput. 1980; 18:709–718.14. Dell'Italia LJ, Walsh RA. Application of a time varying elastance model right ventricular performance in man. Cardiovasc Res. 1988; 22:864–874.15. Negroni JA, Lascano EC. A cardiac muscle model relating sarcomere dynamics to calcium kinetics. J Mol Cell Cardiol. 1996; 28:915–929.16. Nygren A, Fiset C, Firek L, Clark JW, Lindblad DS, Clark RB, Giles WR. Mathematical model of an adult human atrial cell: the role of K+ currents in repolarization. Circ Res. 1998; 82:63–81.17. ten Tusscher KH, Noble D, Noble PJ, Panfilov AV. A model for human ventricular tissue. Am J Physiol Heart Circ Physiol. 2004; 286:H1573–H1589.18. Matsuoka S, Sarai N, Kuratomi S, Ono K, Noma A. Role of individual ionic current systems in ventricular cells hypothesized by a model study. Jpn J Physiol. 2003; 53:105–123.19. Heldt T, Shim EB, Kamm RD, Mark RG. Computational modeling of cardiovascular response to orthostatic stress. J Appl Physiol. 2002; 92:1239–1254.20. Avolio AP. Multi-branched model of the human arterial system. Med Biol Eng Comput. 1980; 18:709–718.21. Izzo JL Jr, Shykoff BE. Arterial stiffness: clinical relevance, measurement, and treatment. Rev Cardiovasc Med. 2001; 2:29–34.22. Gozna ER, Marble AE, Shaw AJ, Winter DA. Mechanical properties of the ascending thoracic aorta of man. Cardiovasc Res. 1973; 7:261–265.23. Lehmann ED, Parker JR, Hopkins KD, Taylor MG, Gosling RG. Validation and reproducibility of pressure-corrected aortic distensibility measurements using pulse-wave-velocity Doppler ultrasound. J Biomed Eng. 1993; 15:221–228.24. Stewart AD, Millasseau SC, Kearney MT, Ritter JM, Chowienczyk PJ. Effects of inhibition of basal nitric oxide synthesis on carotid-femoral pulse wave velocity and augmentation index in humans. Hypertension. 2003; 42:915–918.25. Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, Umans JG, Calhoun D, Howard BV. High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol. 2009; 54:1730–1734.26. Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009; 27:461–467.27. Rochette L, Tatou E, Maupoil V, Zeller M, Cottin Y, Jazayeri S, Brenot R, Girard C, David M, Vergely C. Atrial and vascular oxidative stress in patients with heart failure. Cell Physiol Biochem. 2011; 27:497–502.28. Kashif FM, Verghese GC, Novak V, Czosnyka M, Heldt T. Model-based noninvasive estimation of intracranial pressure from cerebral blood flow velocity and arterial pressure. Sci Transl Med. 2012; 4:129ra44.29. Landesberg A, Sideman S. Mechanical regulation of cardiac muscle by coupling calcium kinetics with cross-bridge cycling: a dynamic model. Am J Physiol. 1994; 267:H779–H795.30. Beyar R, Sideman S. Atrioventricular interactions: a theoretical simulation study. Am J Physiol. 1987; 252:H653–H665.31. Ursino M, Innocenti M. Modeling arterial hypotension during hemodialysis. Artif Organs. 1997; 21:873–890.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiovascular dysfunction and liver transplantation
- Acute and Chronic Effects of Smoking on the Arterial Wall Properties and the Hemodynamics in Smokers with Hypertension
- Echocardiographic Assessment of Right Ventriculo-arterial Coupling: Clinical Correlates and Prognostic Impact in Heart Failure Patients Undergoing Cardiac Resynchronization Therapy
- Pulsatile Hemodynamics and Coronary Artery Disease
- Numerical Simulation of the Effect of Sodium Profile on Cardiovascular Response to Hemodialysis