J Korean Med Sci.
2010 Apr;25(4):570-576. 10.3346/jkms.2010.25.4.570.
Differential Expression of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Thioacetamide-Induced Chronic Liver Injury
- Affiliations
-
- 1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea. tripj@snu.ac.kr
- 2Department of Veterinary Lab Animal Medicine & Science, Kangwon National University, Chuncheon, Korea.
- KMID: 1792927
- DOI: http://doi.org/10.3346/jkms.2010.25.4.570
Abstract
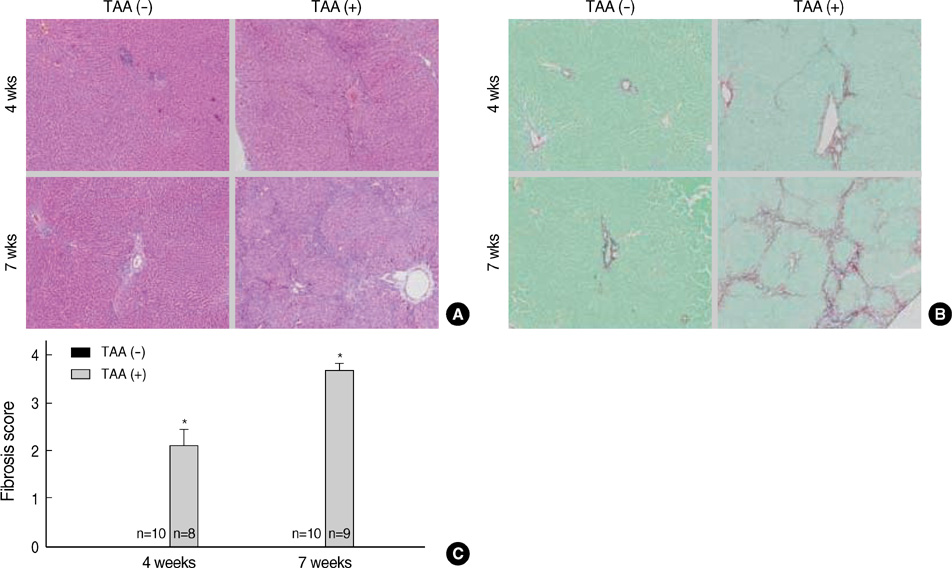
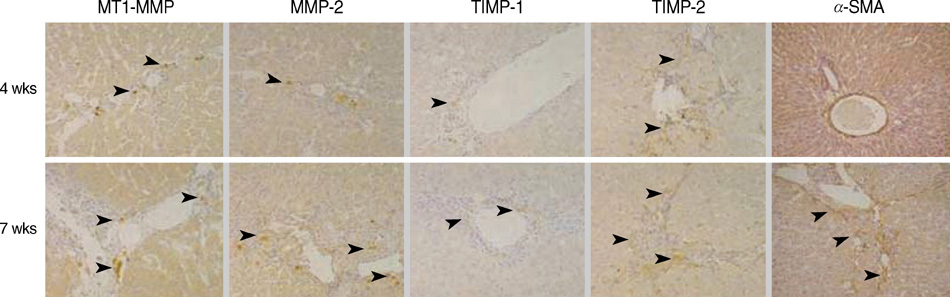
- Hepatic fibrogenesis, a complex process that involves a marked accumulation of extracellular matrix components, activation of cells capable of producing matrix materials, cytokine release, and tissue remodeling, is regulated by matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs). The MMP-TIMP balance can regulate liver fibrogenesis. The aim of this study was to evaluate the expression patterns of MMPs and TIMPs during thioacetamide (TAA)-induced liver fibrogenesis. Chronic liver injury was induced with TAA (200 mg/kg i.p.) for 4 or 7 weeks in male Sprague-Dawley rats. Hepatic injury and fibrosis were assessed by hematoxylin-eosin (H&E) staining, and collagen deposition was confirmed by Sirius Red staining. The level of hepatic injury was quantified by serological analysis. The transcriptional and translational levels of alpha-smooth muscle actin (alpha-SMA), MMPs, and TIMPs in the liver were measured by Western blotting, RT-PCR, and immunohistochemistry. MMP, TIMP, and alpha-SMA were observed along fibrotic septa and portal spaces around the lobules. TAA treatment increased transcription of both MMPs and TIMPs, but only TIMPs showed increased translation. The dominant expression of TIMPs may regulate the function of MMPs to maintain liver fibrosis induced by TAA.
Keyword
MeSH Terms
-
Animals
Collagen/metabolism
Extracellular Matrix/chemistry/metabolism
*Liver Cirrhosis/chemically induced/metabolism/pathology
Male
Matrix Metalloproteinases/genetics/*metabolism
Rats
Rats, Sprague-Dawley
Thioacetamide/*toxicity
Tissue Inhibitor of Metalloproteinases/genetics/*metabolism
Tissue Inhibitor of Metalloproteinases
Thioacetamide
Collagen
Matrix Metalloproteinases
Figure
Reference
-
1. Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. J Biol Chem. 1995. 270:5331–5338.
Article2. Herbst H, Wege T, Milani S, Pellegrini G, Orzechowski HD, Bechstein WO, Neuhaus P, Gressner AM, Schuppan D. Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol. 1997. 150:1647–1659.3. Takahara T, Furui K, Yata Y, Jin B, Zhang LP, Nambu S, Sato H, Seiki M, Watanabe A. Dual expression of matrix metalloproteinase-2 and membrane-type 1 matrix metalloproteinase in fibrotic human livers. Hepatology. 1997. 26:1521–1529.4. Knäuper V, Will H, López-Otin C, Smith B, Atkinson SJ, Stanton H, Hembry RM, Murphy G. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996. 271:17124–17131.5. Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, Schadevan-Westrum S, Crabbe T, Clements J, d'Ortho MP, Murphy G. The TIMP2 membrane type 1 metalloproteinase "Receptor" regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998. 273:871–880.6. Sato H, Kinoshita T, Takino T, Nakayama K, Seiki M. Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett. 1996. 393:101–104.
Article7. Krane SM, Byrne MH, Lemaître V, Henriet P, Jeffrey JJ, Witter JP, Liu X, Wu H, Jaenisch R, Eeckhout Y. Different collagenase gene products have different roles in degradation of type I collagen. J Biol Chem. 1996. 271:28509–28515.
Article8. Benyon RC, Arthur MJ. Extracellular matrix degradation and the role hepatic stellate cells. Semin Liver Dis. 2001. 21:373–384.9. Kim TH, Mars WM, Stolz DB, Michalopoulos GK. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000. 31:75–82.
Article10. Lindquist JN, Parsons CJ, Stefanovic B, Brenner DA. Regulation of α1(I) collagen messenger RNA decay by interaction with αCP at the 3'-untranslated region. J Biol Chem. 2004. 279:23822–23829.11. Nie QH, Duan GR, Luo XD, Xie YM, Luo H, Zhou YX, Pan BR. Expression of TIMP-1 and TIMP-2 in rats with hepatic fibrosis. World J Gastroenterol. 2004. 10:86–90.
Article12. Uchinami H, Seki E, Brenner DA, D'Armiento J. Loss of MMP13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology. 2006. 44:420–429.13. Taras D, Blanc JF, Rullier A, Dugot-Senant N, Laurendeau I, Vidaud M, Rosenbaum J. Pravastatin reduces lung metastasis of rat hepatocellular carcinoma via a coordinated decrease of MMP expression and activity. J Hepatol. 2007. 46:69–76.
Article14. Iredale JP. Cirrhosis: new research provides a basis for rational and targeted treatments. BMJ. 2003. 327:143–147.
Article15. Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991. 13:372–374.
Article16. Liotta LA, Stetler-Stevenson WG. Metalloproteinases and cancer invasion. Semin Cancer Biol. 1990. 1:99–106.17. Tsukamoto H, Matsuoka M, French SW. Experimental models of hepatic fibrosis: a review. Semin Liver Dis. 1990. 10:56–65.
Article18. Waters NJ, Waterfield CJ, Farrant RD, Holmes E, Nicholson JK. Metabonomic deconvolution of embedded toxicity: application to thioacetamide hepato- and nephrotoxicity. Chem Res Toxicol. 2005. 18:639–654.
Article19. Wang CH, Lee TH, Lu CN, Chou WY, Hung KS, Concejero AM, Jawan B. Electroporative α-MSH gene transfer attenuates thioacetamide-induced murine hepatic fibrosis by MMP and TIMP modulation. Gene Therapy. 2006. 13:1000–1009.
Article20. Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998. 21:75–80.21. Cheong JY, Cho SW, Lee JA, Lee KJ, Wang HJ, Lee JE, Kim JH. Matrix metalloproteinase-3 genotypes influence recovery from hepatitis B virus infection. J Korean Med Sci. 2008. 23:61–65.
Article22. Milani S, Herbst H, Schuppan D, Grappone C, Pellegrini G, Pinzani M, Casini A, Calabró A, Ciancio G, Stefanni F. Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol. 1994. 144:528–537.23. Iredale JP, Murphy G, Hembry RM, Friedman SL, Arthur MJ. Human hepatic lipocytes synthesize tissue inhibitor of metalloproteinases-1. Implications for regulation of matrix degradation in liver. J Clin Invest. 1992. 90:282–287.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Tissue Levels of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Gastric Cancer
- Expression of Matrix Metalloproteinases and Its Inhibitor in Gastric Adenocarcinoma
- Role of Matrix Metalloproteinase-9 in Asthma
- Expression of mRNA for matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gingival and periodontal ligament fibroblasts treated with lipopolysaccharide from Prevotella intermedia
- The effect of periodontal flap surgery on Matrix metalloproteinases (MMPs) and Tissue inhibitors of matrix metalloproteinase-1 (TIMP-1) levels in gingival crevicular fluids of periodontitis patients