J Korean Med Sci.
2011 Oct;26(10):1310-1315. 10.3346/jkms.2011.26.10.1310.
Changes in Renal Function after Different Tandem Hematopoietic Stem-cell Transplantation Approaches in Patients with Multiple Myeloma
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Yeungnam University Hospital, Daegu, Korea.
- 2Division of Nephrology, Department of Internal Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. cheolwhee@hanmail.net
- 3Division of Hematology, Department of Internal Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea.
- KMID: 1785988
- DOI: http://doi.org/10.3346/jkms.2011.26.10.1310
Abstract
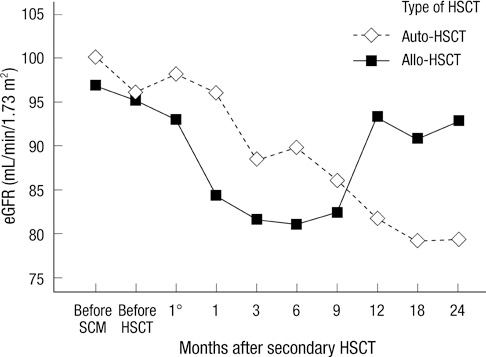
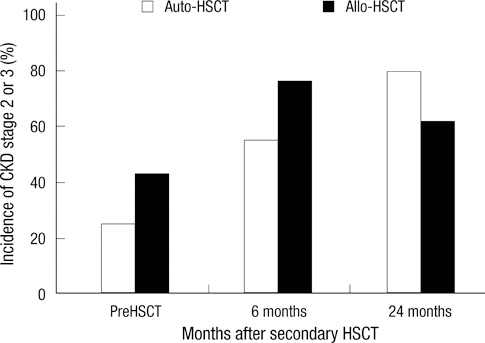
- This study was done to observe the alteration of the estimated glomerular filtration rate (eGFR) in multiple myeloma patients according to type of tandem hematopoietic stem cell transplantation (HSCT). Forty-one patients were enrolled in this study. Twenty patients underwent autologous HSCT (auto-HSCT) and 21 patients underwent allogeneic HSCT (allo-HSCT). The changes in eGFR after the two tandem HSCT modalities were different between the two groups, according to the donor of stem cells (P = 0.016). In the auto-HSCT group, the eGFR, recorded 12 months after secondary HSCT, was significantly decreased compared with the eGFR recorded before stem cell mobilization (P = 0.005). Although there was no significant difference, the trend showed that the eGFR after allo-HSCT decreased from the previous HSCT until a month after secondary HSCT. In addition, after 6 months of secondary HSCT, the eGFR recovered to the level recorded prior to the HSCT (P = 0.062). This difference may be due to total body irradiation, a calcineurin inhibitor, or maintemance therapy. Changes in renal function would be monitored closely for these patients. The recovery of the eGFR would be a main focus for the patients treated with the total body irradiation or the calcineurin inhibitor, a progressive decline of the eGFR would be also crucial for the patients treated with maintenance therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Knudsen LM, Hippe E, Hjorth M, Holmberg E, Westin J. Renal function in newly diagnosed multiple myeloma: a demographic study of 1353 patients. Eur J Haematol. 1994. 53:207–212.2. Chan GS, Lam MF, Au WY, Chim S, Tse KC, Lo SH, Fung SH, Lai KN, Chan KW. Clinicopathologic analysis of renal biopsies after haematopoietic stem cell transplantation. Nephrology. 2008. 13:322–330.3. Attal M, Harousseau JL, Facon T, Guihot F, Doyen C, Fuzibet JG, Monconduit M, Hulin C, Caillot D, Bouabdallah R, Voillat L, Sotto JJ, Grosbois B, Bataille R. InterGroupe Francophone du Myélome. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003. 349:2495–2502.4. Parikh GC, Amjad AI, Saliba RM, Kazmi SM, Khan ZU, Lahoti A, Hosing C, Mendoza F, Qureshi SR, Weber DM, Wang M, Popat U, Alousi AM, Champlin RE, Giralt SA, Qazilbash MH. Autologous hematopoietic stem cell transplantation may reverse renal failure in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009. 15:812–816.5. Parikh CR, McSweeney P, Schrier RW. Acute renal failure independently predicts mortality after myeloablative allogeneic hematopoietic cell transplant. Kidney Int. 2005. 67:1999–2005.6. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment and survival. Cancer. 1975. 36:842–854.7. Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000. 11:155A.8. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002. 39:2 Suppl 1. S1–S266.9. Eom KS, Min CK, Lee S, Kim YJ, Kim SY, Cho SG, Lee JW, Min WS, Kim CC. Pre-transplant disease status is important for an improved outcome of the second stem cell transplantation in the myeloma patients receiving the first autologous stem cell transplantation. Korean J Hematol. 2006. 41:36–40.10. Jung SK, Kim MS, Lim JH, Kim YG, Han KJ, Min CK, Min WS. Serum free light chains for diagnosis and follow-up of multiple myeloma. Korean J Lab Med. 2008. 28:169–173.11. Alexanian R, Barlogie B, Dixon D. Renal failure in multiple myeloma: pathogenesis and prognostic implications. Arch Intern Med. 1990. 150:1693–1695.12. Dimopoulos MA, Kastritis E, Rosinol L, Bladé J, Ludwig H. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008. 22:1485–1493.13. Tricot G, Alberts DS, Johnson C, Roe DJ, Dorr RT, Bracy D, Vesole DH, Jagannath S, Meyers R, Barlogie B. Safety of autotransplants with high-dose melphalan in renal failure: a pharmacokinetic and toxicity study. Clin Cancer Res. 1996. 2:947–952.14. Nair B, Waheed S, Szymonifka J, Shaughnessy JD Jr, Crowley J, Barlogie B. Immunoglobulin isotypes in multiple myeloma: laboratory correlates and prognostic implications in total therapy protocols. Br J Haematol. 2009. 145:134–137.15. Lokhorst H, Einsele H, Vesole D, Bruno B, San Miguel J, Pérez-Simon JA, Kröger N, Moreau P, Gahrton G, Gasparetto C, Giralt S, Bensinger W. International Myeloma Working Group. International Myeloma Working Group consensus statement regarding the current status of allogeneic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2010. 28:4521–4530.16. Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, Giaccone L, Sorasio R, Omedé P, Baldi I, Bringhen S, Massaia M, Aglietta M, Levis A, Gallamini A, Fanin R, Palumbo A, Storb R, Ciccone G, Boccadoro M. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007. 356:1110–1120.17. Harousseau JL. Maintenance treatment in multiple myeloma. Ann Oncol. 2008. 19:Suppl 4. iv54–iv55.18. Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Owen RG, Feyler S, Ashcroft AJ, Ross F, Byrne J, Roddie H, Rudin C, Cook G, Jackson GH, Child JA. National Cancer Research Institute Haematological Oncology Clinical Study Group. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010. 376:1989–1999.19. Mihelic R, Kaufman JL, Lonial S. Maintenance therapy in multiple myeloma. Leukemia. 2007. 21:1150–1157.20. Berenguer M. Treatment of chronic hepatitis C in hemodialysis patients. Hepatology. 2008. 48:1690–1699.21. Diel IJ, Weider R, Köppler H, Antràs L, Smith M, Green J, Wintfeld N, Neary M, Duh MS. Risk of renal impairment after treatment with ibandronate versus zoledronic acid: a retrospective medical records review. Support Care Cancer. 2009. 17:719–725.22. Izzedine H, Launay-Vacher V, Deray G. Thalidomide for the nephrologist. Nephrol Dial Transplant. 2005. 20:2011–2012.23. Jones SG, Dolan G, Lengyel K, Myers B. Severe increase in creatinine with hypocalcemia in thalidomide-treated myeloma patients receiving zoledronic acid infusions. Br J Haematol. 2002. 119:576–577.24. Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplantation: epidemiology, pathogenesis, and treatment. J Am Soc Nephrol. 2006. 17:1995–2005.25. Borg M, Hughes T, Horvath N, Rice M, Thomas AC. Renal toxicity after total body irradiation. Int J Radiat Oncol Biol Phys. 2002. 54:1165–1173.26. Kahan BD. Cyclosporine. N Engl J Med. 1989. 321:1725–1738.27. Nunes R, Pasos-Coelho JL, Miranda N, Nave M, da Costa FL, Abecasis M. Reversible acute renal failure following single administration of fludarabine. Bone Marrow Transplant. 2004. 33:671.28. Brukamp K, Doyle AM, Bloom RD, Bunin N, Tomaszewski JE, Cizman B. Nephrotic syndrome after hematopoietic cell transplantation: do glomerular lesions represent renal graft-versus-host disease? Clin J Am Soc Nephrol. 2006. 1:685–694.29. Lee CK, Zangari M, Barlogie B, Fassas A, van Rhee F, Thertulien R, Talamo G, Muwalla F, Anaissie E, Hollmig K, Tricot G. Dialysis-dependent renal failure in patients with myeloma can be reversed by high-dose myeloablative therapy and autotransplant. Bone Marrow Transplant. 2004. 33:823–828.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Cerebral Toxoplasmosis Following Tandem Autologous Stem Cell Transplantation in a Multiple Myeloma Patient
- Reverse Seroconversion of Hepatitis B following Allogenic Hematopoietic Stem Cell Transplantation from a Hepatitis Immune Donor in a Multiple Myeloma Patient
- HBV reactivation in a HBsAg-negative patient with multiple myeloma treated with prednisolone maintenance therapy after autologous HSCT
- Successful Chemotherapy Following Autologous Stem Cell Transplantation in Multiple Myeloma and Multi-organ Dysfunction with Infiltration of Eosinophils: A Case Report
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation