J Korean Med Sci.
2011 Oct;26(10):1270-1276. 10.3346/jkms.2011.26.10.1270.
Intratumoral Administration of Secondary Lymphoid Chemokine and Unmethylated Cytosine-phosphorothioate-guanine Oligodeoxynucleotide Synergistically Inhibits Tumor Growth in Vivo
- Affiliations
-
- 1Laboratory of Immunology, Transplantation Research Institute, Seoul, Korea. dlee5522@snu.ac.kr
- 2Interdisciplinary Program of Tumor Biology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1785982
- DOI: http://doi.org/10.3346/jkms.2011.26.10.1270
Abstract
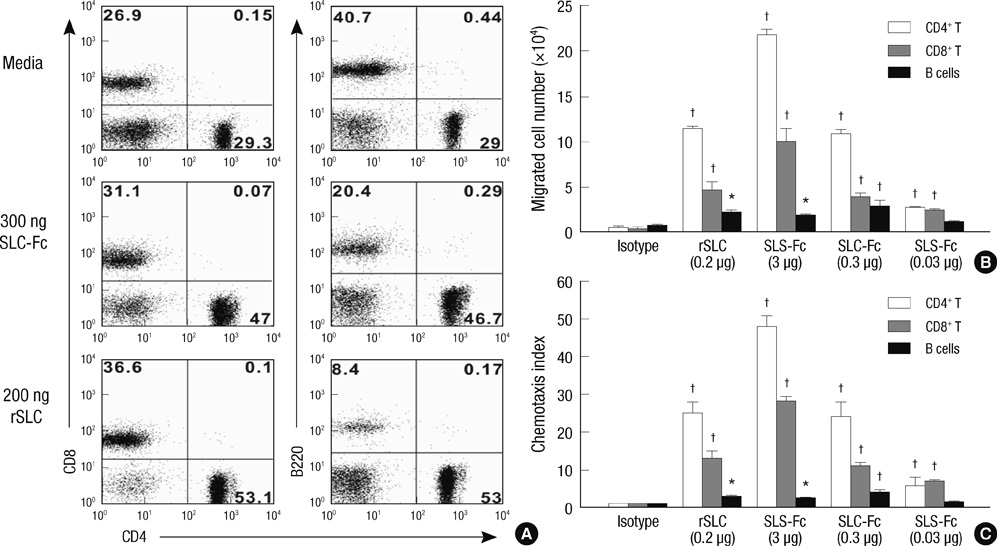
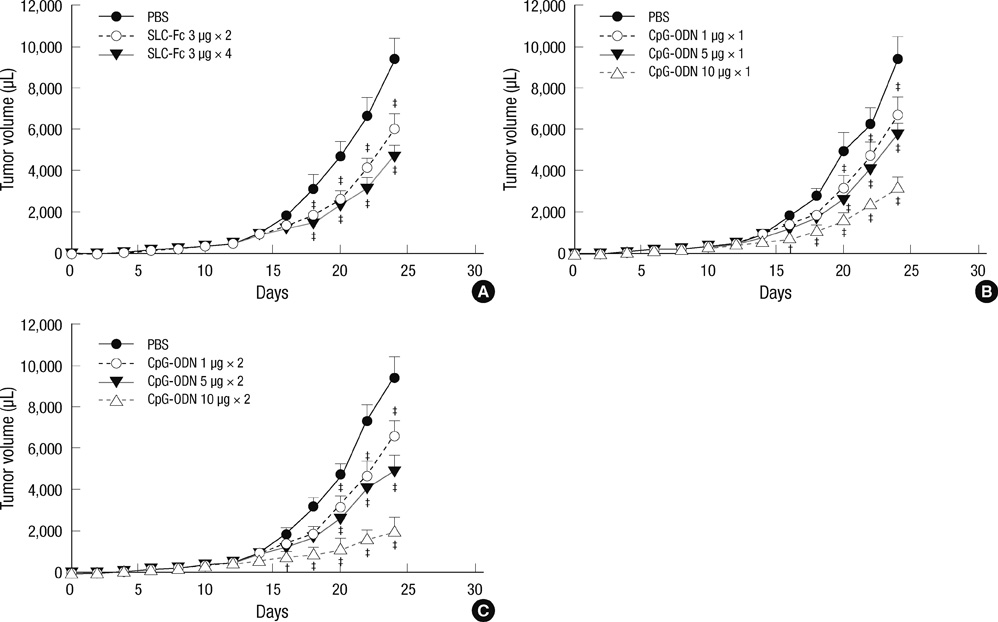
- Secondary lymphoid tissue chemokine (SLC), which is expressed in T cell zones of secondary lymphoid organs, including the spleen and lymph nodes, strongly recruits both T lymphocytes and mature dendritic cells. As appropriate interaction of tumor-specific T cells and mature dendritic cells, equipped with tumor antigens, is a prerequisite for effective T cell immunity against established tumors, we mobilized lymphocytes and dendritic cells to tumor sites by intratumoral injection of secondary lymphoid tissue chemokine-Fc (SLC-Fc) fusion protein using the B16F10 murine melanoma model. Activation of dendritic cells, another prerequisite for the effective activation of naive tumor-specific T cells, was achieved by the addition of immunostimulatory cytosine-phosphorothioate-guanine oligodeoxynucleotide (CpG-ODN) into the tumor site. Intratumoral administration of SLC-Fc or CpG-ODN revealed antitumor effects against B16F10 murine melanoma grown in the subcutaneous space. Co-treatment of SLC-Fc and CpG-ODN displayed synergistic effects in reducing the tumor size. The synergistic antitumor effect in co-treatment group was correlated with the synergistic/additive increase in the infiltration of CD4+ T cells and CD11c+ dendritic cells in the tumor mass compared to the single treatment groups. These results suggest that the combined use of chemokines and adjuvant molecules may be a possible strategy in clinical tumor immunotherapy.
Keyword
MeSH Terms
-
Animals
Antigens, CD11c/immunology
CD4-Positive T-Lymphocytes/immunology
Cell Line, Tumor
Cell Proliferation/drug effects
Chemokine CCL21/*administration & dosage/pharmacology
Chemotaxis, Leukocyte
Dendritic Cells/immunology/metabolism
Immunotherapy
Injections, Intralesional
Melanoma, Experimental/*immunology/*therapy
Mice
Mice, Inbred C57BL
Oligodeoxyribonucleotides/*administration & dosage/pharmacology
T-Lymphocytes/immunology/metabolism
Figure
Reference
-
1. Slingluff CL Jr, Chianese-Bullock KA, Bullock TN, Grosh WW, Mullins DW, Nichols L, Olson W, Petroni G, Smolkin M, Engelhard VH. Immunity to melanoma antigens: from self-tolerance to immunotherapy. Adv Immunol. 2006. 90:243–295.2. Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006. 311:17–58.3. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004. 4:540–550.4. Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004. 22:891–928.5. Randall TD, Carragher DM, Rangel-Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol. 2008. 26:627–650.6. Cyster JG. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J Exp Med. 1999. 189:447–450.7. Chan VW, Kothakota S, Rohan MC, Panganiban-Lustan L, Gardner JP, Wachowicz MS, Winter JA, Williams LT. Secondary lymphoid-tissue chemokine (SLC) is chemotactic for mature dendritic cells. Blood. 1999. 93:3610–3616.8. Yoshida R, Nagira M, Kitaura M, Imagawa N, Imai T, Yoshie O. Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J Biol Chem. 1998. 273:7118–7122.9. Jenh CH, Cox MA, Kaminski H, Zhang M, Byrnes H, Fine J, Lundell D, Chou CC, Narula SK, Zavodny PJ. Cutting edge: species specificity of the CC chemokine 6Ckine signaling through the CXC chemokine receptor CXCR3: human 6Ckine is not a ligand for the human or mouse CXCR3 receptors. J Immunol. 1999. 162:3765–3769.10. Tokunaga T, Yamamoto H, Shimada S, Abe H, Fukuda T, Fujisawa Y, Furutani Y, Yano O, Kataoka T, Sudo T. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst. 1984. 72:955–962.11. Kleeberg UR, Engel E, Israels P, Bröcker EB, Tilgen W, Kennes C, Gérard B, Lejeune F, Glabbeke MV, Lentz MA. Palliative therapy of melanoma patients with fotemustine. Inverse relationship between tumor load and treatment effectiveness. A multicentre phase II trial of the EORTC-Melanoma Cooperative Group (MCG). Melanoma Res. 1995. 5:195–200.12. Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A toll-like receptor recognizes bacterial DNA. Nature. 2000. 408:740–745.13. Jones TR, Obaldia N 3rd, Gramzinski RA, Charoenvit Y, Kolodny N, Kitov S, Davis HL, Krieg AM, Hoffman SL. Synthetic oligodeoxynucleotides containing CpG motifs enhance immunogenicity of a peptide malaria vaccine in Aotus monkeys. Vaccine. 1999. 17:3065–3071.14. Vabulas RM, Pircher H, Lipford GB, Häcker H, Wagner H. CpG-DNA activates in vivo T cell epitope presenting dendritic cells to trigger protective antiviral cytotoxic T cell responses. J Immunol. 2000. 164:2372–2378.15. Jakob T, Walker PS, Krieg AM, Udey MC, Vogel JC. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol. 1998. 161:3042–3049.16. Gray RJ, Pockaj BA, Kirkwood JM. An update on adjuvant interferon for melanoma. Cancer Control. 2002. 9:16–21.17. Lo JC, Chin RK, Lee Y, Kang HS, Wang Y, Weinstock JV, Banks T, Ware CF, Franzoso G, Fu YX. Differential regulation of CCL21 in lymphoid/non-lymphoid tissues for effectively attracting T cells to peripheral tissues. J Clin Invest. 2003. 112:1495–1505.18. Krautwald S, Ziegler E, Förster R, Ohl L, Amann K, Kunzendorf U. Ectopic expression of CCL19 impairs alloimmune response in mice. Immunology. 2004. 112:301–309.19. Yang SH, Oh TK, Kim ST. Increased anti-tumor effect by a combination of HSV thymidine kinase suicide gene therapy and interferon-gamma/GM-CSF cytokine gene therapy in CT26 tumor model. J Korean Med Sci. 2005. 20:932–937.20. Park JN, Suh C, Yang J, Park JS, Park KU, Min YJ, Kim HJ, Kim YH, Kim SH. Active immunization using dendritic cells mixed with tumor cells inhibits the growth of lymphomas. J Korean Med Sci. 2003. 18:372–380.21. Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, Zinkernagel R, Aguzzi A. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med. 2004. 10:187–192.22. Dubinett SM, Lee JM, Sharma S, Mulé JJ. Chemokines: can effector cells be redirected to the site of the tumor? Cancer J. 2010. 16:325–335.23. Ruffini PA, Morandi P, Cabioglu N, Altundag K, Cristofanilli M. Manipulating the chemokine-chemokine receptor network to treat cancer. Cancer. 2007. 109:2392–2404.24. Zhang T, Somasundaram R, Berencsi K, Caputo L, Rani P, Guerry D, Furth E, Rollins BJ, Putt M, Gimotty P, Swoboda R, Herlyn M, Herlyn D. CXC chemokine ligand 12 (stromal cell-derived factor 1 alpha) and CXCR4-dependent migration of CTLs toward melanoma cells in organotypic culture. J Immunol. 2005. 174:5856–5863.25. Shurin GV, Ferris RL, Tourkova IL, Perez L, Lokshin A, Balkir L, Collins B, Chatta GS, Shurin MR. Loss of new chemokine CXCL14 in tumor tissue is associated with low infiltration by dendritic cells (DC), while restoration of human CXCL14 expression in tumor cells causes attraction of DC both in vitro and in vivo. J Immunol. 2005. 174:5490–5498.26. Thanarajasingam U, Sanz L, Diaz R, Qiao J, Sanchez-Perez L, Kottke T, Thompson J, Chester J, Vile RG. Delivery of CCL21 to metastatic disease improves the efficacy of adoptive T-cell therapy. Cancer Res. 2007. 67:300–308.27. Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, Lebecque S, Fridman WH, Cadranel J. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008. 26:4410–4417.28. Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoid like stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010. 328:749–752.29. Hisada M, Yoshimoto T, Kamiya S, Magami Y, Miyaji H, Yoneto T, Tamada K, Aoki T, Koyanagi Y, Mizuguchi J. Synergistic antitumor effect by coexpression of chemokine CCL21/SLC and costimulatory molecule LIGHT. Cancer Gene Ther. 2004. 11:280–288.30. Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, Schietinger A, Philip M, Schreiber H, Fu YX. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004. 5:141–149.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-proliferative Effect of Engineered Neural Stem Cells Expressing Cytosine Deaminase and Interferon-β against Lymph Node–Derived Metastatic Colorectal Adenocarcinoma in Cellular and Xenograft Mouse Models
- Phase I clinical trial of simultaneous administration of intraperitoneal carboplatin, etoposide and cytosine arabinoside for refractory ovarian carcinoma
- Growth Hormone Receptor Mutation and Partial Growth Hormone Insensitivity in Children with Idiopathic Short Stature
- Adenovirus-Mediated Toxic Gene Therapy Using Cytosine Deaminase and Osteocalcin Promoter for the Treatment of Prostate Cancer
- Effect of Cytosine-phosphate-Guanine Oligodeoxynucleotides on Airway Hyperresponsiveness and Inflammation of Repeated Respiratory Syncytial Virus Infection in a Mouse Model