J Korean Med Sci.
2010 Sep;25(9):1305-1312. 10.3346/jkms.2010.25.9.1305.
High Dose Vitamin D3 Attenuates the Hypocalciuric Effect of Thiazide in Hypercalciuric Rats
- Affiliations
-
- 1Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Gachon University of Medicine and Science, Incheon, Korea.
- 4Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. junephro@snuh.org
- KMID: 1785909
- DOI: http://doi.org/10.3346/jkms.2010.25.9.1305
Abstract
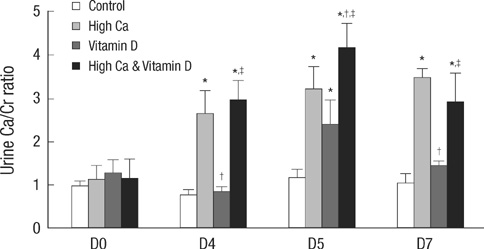
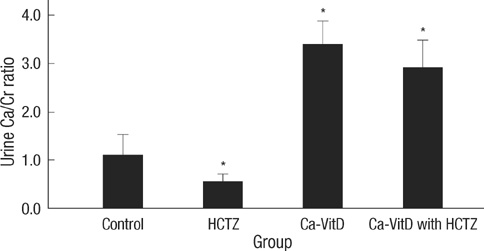
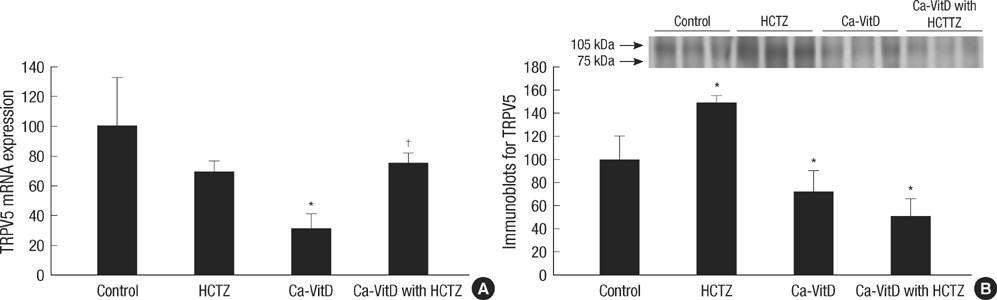
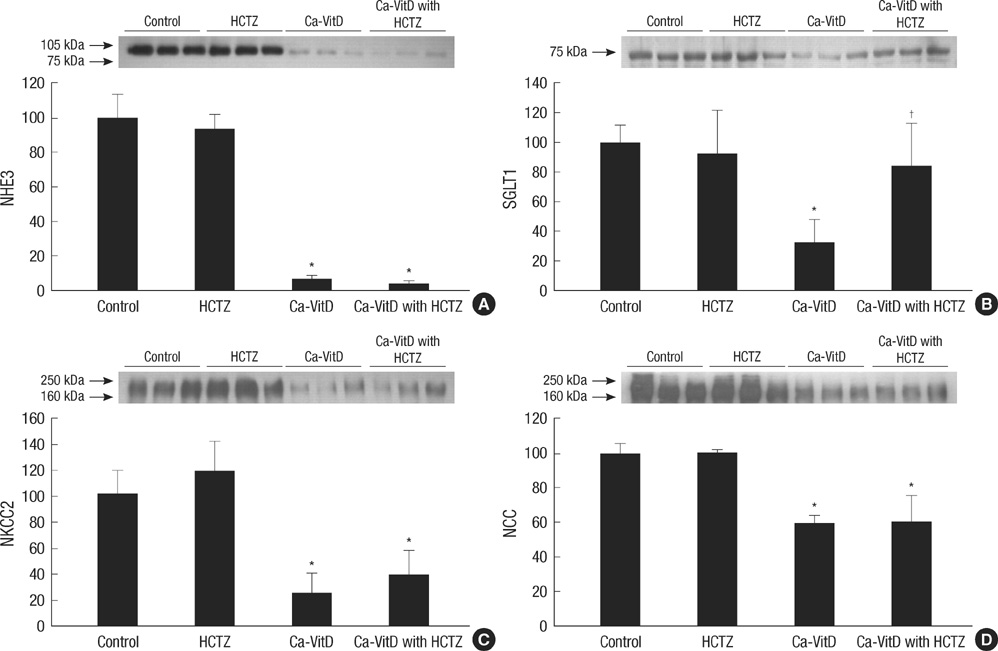
- Thiazide is known to decrease urinary calcium excretion. We hypothesized that thiazide shows different hypocalciuric effects depending on the stimuli causing hypercalciuria. The hypocalciuric effect of hydrochlorothiazide (HCTZ) and the expression of transient receptor potential vanilloid 5 (TRPV5), calbindin-D(28K), and several sodium transporters were assessed in hypercalciuric rats induced by high calcium diet and vitamin D3. Urine calcium excretion and the expression of transporters were measured from 4 groups of Sprague-Dawley rats; control, HCTZ, high calcium-vitamin D, and high calcium-vitamin D with HCTZ groups. HCTZ decreased urinary calcium excretion by 51.4% in the HCTZ group and only 15% in the high calcium-vitamin D with HCTZ group. TRPV5 protein abundance was not changed by HCTZ in the high calcium-vitamin D with HCTZ group compared to the high calcium-vitamin D group. Protein abundance of NHE3, SGLT1, and NKCC2 decreased in the hypercalciuric rats, and only SGLT1 protein abundance was increased by HCTZ in the hypercalciuric rats. The hypocalciuric effect of HCTZ is attenuated in high calcium and vitamin D-induced hypercalciuric rats. This attenuation seems to have resulted from the lack of HCTZ's effect on protein abundance of TRPV5 in severe hypercalciuric condition induced by high calcium and vitamin D.
Keyword
MeSH Terms
-
Animals
Calcium/therapeutic use/urine
Calcium Channels/genetics/metabolism
Cholecalciferol/*toxicity
Hydrochlorothiazide/*therapeutic use
Hypercalciuria/chemically induced/*drug therapy
Rats
Rats, Sprague-Dawley
Sodium Chloride Symporter Inhibitors/*therapeutic use
Sodium-Glucose Transporter 1/genetics/metabolism
Sodium-Hydrogen Antiporter/genetics/metabolism
Sodium-Potassium-Chloride Symporters/genetics/metabolism
TRPV Cation Channels/genetics/metabolism
Figure
Reference
-
1. Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001. 59:2290–2298.
Article2. Lerolle N, Coulet F, Lantz B, Paillard F, Houillier P, Soubrier F, Gattegno B, Jeunemaitre X, Ronco P, Rondeau E. No evidence for point mutations of the calcium-sensing receptor in familial idiopathic hypercalciuria. Nephrol Dial Transplant. 2001. 16:2317–2322.
Article3. Praga M, Alegre R, Hernandez E, Morales E, Dominguez-Gil B, Carreno A, Andres A. Familial microscopic hematuria caused by hypercalciuria and hyperuricosuria. Am J Kidney Dis. 2000. 35:141–145.
Article4. Parekh DJ, Pope JC 4th, Adams MC, Brock JW 3rd. The association of an increased urinary calcium-to-creatinine ratio, and asymptomatic gross and microscopic hematuria in children. J Urol. 2002. 167:272–274.5. Asplin JR, Donahue S, Kinder J, Coe FL. Urine calcium excretion predicts bone loss in idiopathic hypercalciuria. Kidney Int. 2006. 70:1463–1467.
Article6. Costanzo LS, Weiner IM. On the hypocalciuric action of chlorothiazide. J Clin Invest. 1974. 54:628–637.
Article7. Ray WA, Griffin MR, Downey W, Melton LJ 3rd. Long-term use of thiazide diuretics and risk of hip fracture. Lancet. 1989. 1:687–690.
Article8. LaCroix AZ, Wienpahl J, White LR, Wallace RB, Scherr PA, George LK, Cornoni-Huntley J, Ostfeld AM. Thiazide diuretic agents and the incidence of hip fracture. N Engl J Med. 1990. 322:286–290.
Article9. Reid IR, Ames RW, Orr-Walker BJ, Clearwater JM, Horne AM, Evans MC, Murray MA, McNeil AR, Gamble GD. Hydrochlorothiazide reduces loss of cortical bone in normal postmenopausal women: a randomized controlled trial. Am J Med. 2000. 109:362–370.
Article10. Rose BD, Post TW. Proximal tubule: Renal physiology. Clinical physiology of acid-base and electrolyte disorders. 2001. New York: McGraw-Hill;71–103.11. Hoenderop JG, Nilius B, Bindels RJ. Molecular mechanism of active Ca2+ reabsorption in the distal nephron. Annu Rev Physiol. 2002. 64:529–549.12. Jang HR, Kim S, Heo NJ, Lee JH, Kim HS, Nielsen S, Jeon US, Oh YK, Na KY, Joo KW, Han JS. Effects of thiazide on the expression of trpv5, calbindin-d28k, and sodium transporters in hypercalciuric rats. J Korean Med Sci. 2009. 24:Suppl 1. S161–S169.
Article13. Brown AJ, Dusso A, Slatopolsky E. Vitamin D. Am J Physiol. 1999. 277:F157–F175.
Article14. Friedman PA, Gesek FA. Cellular calcium transport in renal epithelia: Measurement, mechanisms, and regulation. Physiol Rev. 1995. 75:429–471.
Article15. Hoenderop JG, Muller D, Van Der Kemp AW, Hartog A, Suzuki M, Ishibashi K, Imai M, Sweep F, Willems PH, Van Os CH, Bindels RJ. Calcitriol controls the epithelial calcium channel in kidney. J Am Soc Nephrol. 2001. 12:1342–1349.
Article16. Frick KK, Bushinsky DA. Molecular mechanisms of primary hypercalciuria. J Am Soc Nephrol. 2003. 14:1082–1095.
Article17. Hoenderop JG, Nilius B, Bindels RJ. Epithelial calcium channels: From identification to function and regulation. Pflugers Arch. 2003. 446:304–308.
Article18. Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ. Molecular identification of the apical Ca2+ channel in 1,25-dihydroxyvitamin d3-responsive epithelia. J Biol Chem. 1999. 274:8375–8378.19. Hoenderop JG, Dardenne O, Van Abel M, Van Der Kemp AW, Van Os CH, St -Arnaud R, Bindels RJ. Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin d3 in 25-hydroxyvitamin d3-1alpha-hydroxylase knockout mice. FASEB J. 2002. 16:1398–1406.20. Renkema KY, Nijenhuis T, van der Eerden BC, van der Kemp AW, Weinans H, van Leeuwen JP, Bindels RJ, Hoenderop JG. Hypervitaminosis d mediates compensatory Ca2+ hyperabsorption in trpv5 knockout mice. J Am Soc Nephrol. 2005. 16:3188–3195.21. Van Abel M, Hoenderop JG, Dardenne O, St Arnaud R, Van Os CH, Van Leeuwen HJ, Bindels RJ. 1,25-dihydroxyvitamin d(3)-independent stimulatory effect of estrogen on the expression of ecac1 in the kidney. J Am Soc Nephrol. 2002. 13:2102–2109.
Article22. Bolt MJ, Cao LP, Kong J, Sitrin MD, Li YC. Vitamin d receptor is required for dietary calcium-induced repression of calbindin-d9k expression in mice. J Nutr Biochem. 2005. 16:286–290.
Article23. Nijenhuis T, Hoenderop JG, Loffing J, van der Kemp AW, van Os CH, Bindels RJ. Thiazide-induced hypocalciuria is accompanied by a decreased expression of Ca2+ transport proteins in kidney. Kidney Int. 2003. 64:555–564.24. Loffing J, Loffing-Cueni D, Hegyi I, Kaplan MR, Hebert SC, Le Hir M, Kaissling B. Thiazide treatment of rats provokes apoptosis in distal tubule cells. Kidney Int. 1996. 50:1180–1190.
Article25. Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. 2005. 115:1651–1658.26. Lee CT, Shang S, Lai LW, Yong KC, Lien YH. Effect of thiazide on renal gene expression of apical calcium channels and calbindins. Am J Physiol Renal Physiol. 2004. 287:F1164–F1170.
Article27. Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Merillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking trpv5. J Clin Invest. 2003. 112:1906–1914.28. Gkika D, Hsu YJ, van der Kemp AW, Christakos S, Bindels RJ, Hoenderop JG. Critical role of the epithelial Ca2+ channel trpv5 in active Ca2+ reabsorption as revealed by trpv5/calbindin-d28k knockout mice. J Am Soc Nephrol. 2006. 17:3020–3027.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Thiazide on the Expression of TRPV5, Calbindin-D28K, and Sodium Transporters in Hypercalciuric Rats
- The Role of The Effects of Vitamin D Supplementation on Bone Mineral Density and Bone Mineral Content in Ovariectomized Rats Compensation in Rats
- Efficacy and safety of vitamin D3 B.O.N intramuscular injection in Korean adults with vitamin D deficiency
- Vitamin D3 regulates cell viability in gastric cancer and cholangiocarcinoma
- The effect of Vitamin D(3) and TGF-beta on the viability of human periodontal ligament cells