J Korean Med Sci.
2009 Jan;24(Suppl 1):S161-S169. 10.3346/jkms.2009.24.S1.S161.
Effects of Thiazide on the Expression of TRPV5, Calbindin-D28K, and Sodium Transporters in Hypercalciuric Rats
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. jshan@snu.ac.kr
- 2Institute of Anatomy, University of Aarhus, The Water and Salt Research Center, Aarhus, Denmark.
- 3Postech Biotech Center, Pohang University of Science & Technology, Pohang, Korea.
- KMID: 1778157
- DOI: http://doi.org/10.3346/jkms.2009.24.S1.S161
Abstract
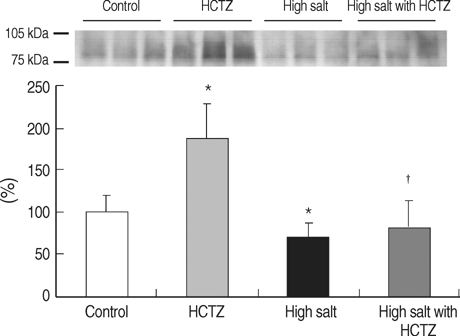
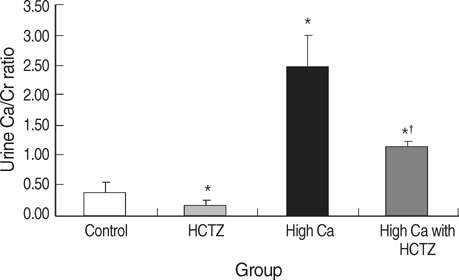
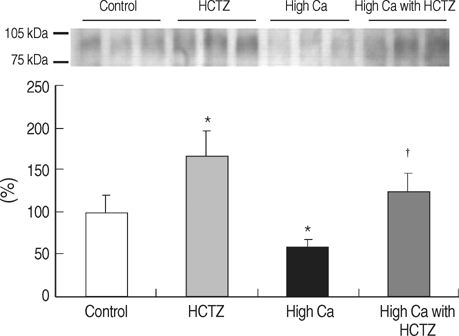
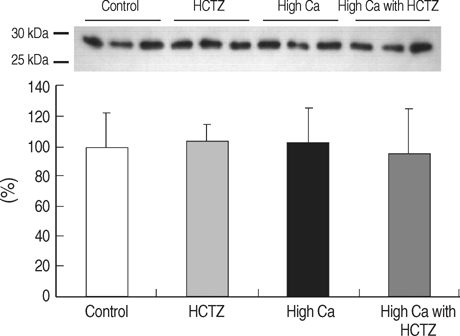
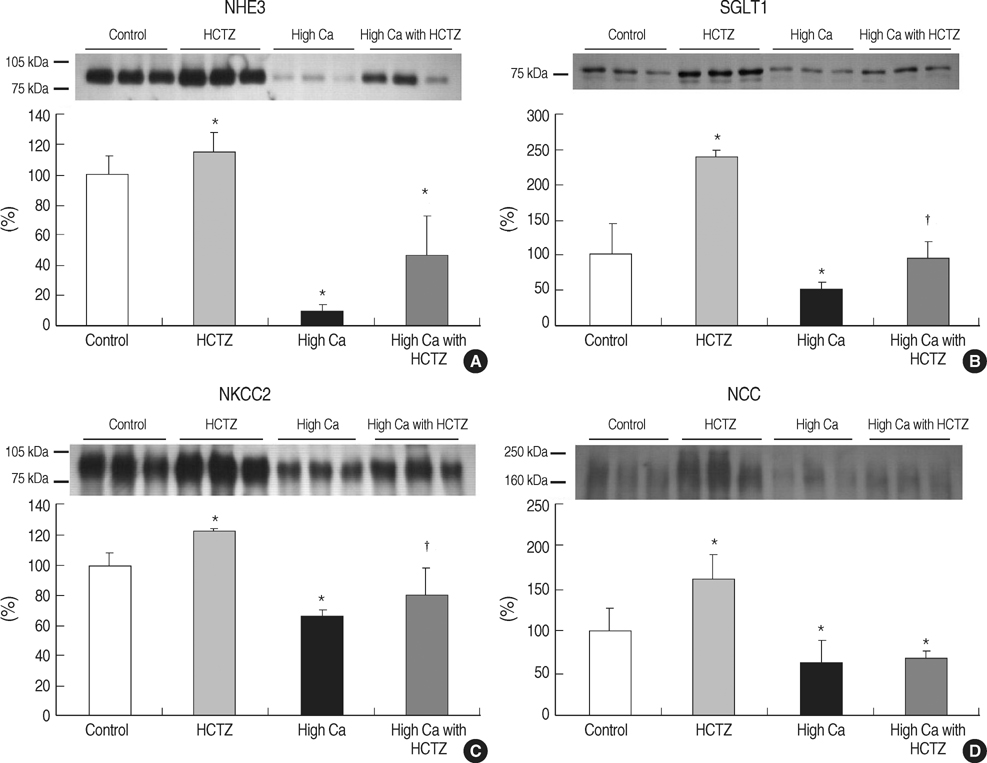
- TRPV5 is believed to play an important role in the regulation of urinary calcium excretion. We assessed the effects of hydrochlorothiazide (HCTZ) on the expression of TRPV5, calbindin-D28K, and several sodium transporters in hypercalciuric rats. Sprague- Dawley rats were divided into 4 groups; control, HCTZ, high salt, and high salt with HCTZ group in experiment 1; control, HCTZ, high calcium (Ca), and high Ca with HCTZ group in experiment 2. To quantitate the expression of TRPV5, calbindin- D28K, and sodium transporters, western blotting was performed. In both experiments, HCTZ significantly decreased urinary calcium excretion. TRPV5 protein abundance decreased in all hypercalciuric rats, and restored by HCTZ in both high salt with HCTZ and high Ca with HCTZ group. Calbindin-D28K protein abundance increased in the high salt and high salt with HCTZ groups, but did not differ among groups in experiment 2. Protein abundance of NHE3 and NKCC2 decreased in all hypercalciuric rats, and were restored by HCTZ in only high Ca-induced hypercalciuric rats. In summary, protein abundance of TRPV5, NHE3, and NKCC2 decreased in all hypercalciuric rats. The hypocalciuric effect of HCTZ is associated with increased protein abundance of TRPV5 in high salt or calcium diet-induced hypercalciuric rats.
Keyword
MeSH Terms
-
Animals
Biological Transport
Calcium/urine
Calcium Channels/chemistry
Calcium-Binding Protein, Vitamin D-Dependent/*biosynthesis
Hydrochlorothiazide/pharmacology
Hypercalciuria/*therapy
Male
Models, Biological
Rats
Rats, Sprague-Dawley
Sodium/*metabolism
Sodium-Hydrogen Antiporter/chemistry
Sodium-Potassium-Chloride Symporters/metabolism
TRPV Cation Channels/*biosynthesis/chemistry
Thiazides/*pharmacology
Figure
Cited by 1 articles
-
High Dose Vitamin D3 Attenuates the Hypocalciuric Effect of Thiazide in Hypercalciuric Rats
Hye Ryoun Jang, Jay Wook Lee, Sejoong Kim, Nam Ju Heo, Jeong Hwan Lee, Hyo Sang Kim, Ji Yong Jung, Yun Kyu Oh, Ki Young Na, Jin Suk Han, Kwon Wook Joo
J Korean Med Sci. 2010;25(9):1305-1312. doi: 10.3346/jkms.2010.25.9.1305.
Reference
-
1. Suki W. Calcium transport in the nephron. Am J Physiol (Lond). 1979. 237:F1–F6.
Article2. Rose Burton David, Post Theodore W.. Proximal tubule: renal physiology. Clinical physiology of acid-base and electrolyte disorders. 2001. New York: McGraw-Hill;71–103.3. Hoenderop JG, Nilius B, Bindels RJ. Molecular mechanism of active ca2+ reabsorption in the distal nephron. Annu Rev Physiol. 2002. 64:529–549.4. Hoenderop JG, Nilius B, Bindels RJ. Epithelial calcium channels: from identification to function and regulation. Pflugers Arch. 2003. 446:304–308.
Article5. Hoenderop JG, Hartog A, Stuiver M, Doucet A, Willems PH, Bindels RJ. Localization of the epithelial ca (2+) channel in rabbit kidney and intestine. J Am Soc Nephrol. 2000. 11:1171–1178.6. Bataille P, Fardellone P, Ghazali A, Cayrolle G, Hottelart C, Achard JM, Fournier A. Pathophysiology and treatment of idiopathic hypercalciuria. Curr Opin Rheumatol. 1998. 10:373–388.
Article7. Lemann J Jr, Gray RW. Idiopathic hypercalciuria. J Urol. 1989. 141:715–718.
Article8. Albright F HP, Benedict PH, Forebes AP. Idiopathic hypercalciuria: a preliminary report. Proc R Soc Med. 1953. 46:1077–1081.9. Backman U, Danielson BG, Johansson G, Ljunghall S, Wikstrom B. Incidence and clinical importance of renal tubular defects in recurrent renal stone formers. Nephron. 1980. 25:96–101.
Article10. Martinez ME, Villa E, Vazquez Martul M, Sanchez-Cabezudo MJ, Sanchez JA, Villa JR. Influence of calcium intake on calcitriol levels in idiopathic hypercalciuria in children. Nephron. 1993. 65:36–39.
Article11. Costanzo LS, Weiner IM. On the hypocalciuric action of chlorothiazide. J Clin Invest. 1974. 54:628–637.
Article12. Ray WA, Griffin MR, Downey W, Melton LJ 3rd. Long-term use of thiazide diuretics and risk of hip fracture. Lancet. 1989. 1:687–690.
Article13. LaCroix AZ, Wienpahl J, White LR, Wallace RB, Scherr PA, George LK, Cornoni-Huntley J, Ostfeld AM. Thiazide diuretic agents and the incidence of hip fracture. N Engl J Med. 1990. 322:286–290.
Article14. Reid IR, Ames RW, Orr-Walker BJ, Clearwater JM, Horne AM, Evans MC, Murray MA, McNeil AR, Gamble GD. Hydrochlorothiazide reduces loss of cortical bone in normal postmenopausal women: a randomized controlled trial. Am J Med. 2000. 109:362–370.
Article15. Frick KK, Bushinsky DA. Molecular mechanisms of primary hypercalciuria. J Am Soc Nephrol. 2003. 14:1082–1095.
Article16. Bushinsky DA. Greenberg , editor. Disorders of calcium and phosphorus homeostasis. Primer on kidney diseases. 2001. San Diego: academic Press;107–115.17. Scheinman SJ, Guay-Woodford LM, Thakker RV, Warnock DG. Genetic disorders of renal electrolyte transport. N Engl J Med. 1999. 340:1177–1187.
Article18. Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev. 2000. 80:277–313.
Article19. Loffing J, Loffing-Cueni D, Valderrabano V, Klausli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol. 2001. 281:F1021–F1027.20. Eladari D, Leviel F, Pezy F, Paillard M, Chambrey R. Rat proximal nhe3 adapts to chronic acid-base disorders but not to chronic changes in dietary nacl intake. Am J Physiol Renal Physiol. 2002. 282:F835–F843.21. Ecelbarger CA, Terris J, Hoyer JR, Nielsen S, Wade JB, Knepper MA. Localization and regulation of the rat renal na(+)-k(+)-2cl-cotransporter, bsc-1. Am J Physiol. 1996. 271:F619–F628.22. Gkika D, Hsu YJ, van der Kemp AW, Christakos S, Bindels RJ, Hoenderop JG. Critical role of the epithelial ca2+ channel trpv5 in active ca2+ reabsorption as revealed by trpv5/calbindin-d28k knockout mice. J Am Soc Nephrol. 2006. 17:3020–3027.
Article23. Kleeman CR, Bohannan J, Bernstein D, Ling S, Maxwell MH. Effect of variations in sodium intake on calcium excretion in normal humans. Proc Soc Exp Biol Med. 1964. 115:29–32.
Article24. Phillips MJ, Cooke JN. Relation between urinary calcium and sodium in patients with idiopathic hypercalciuria. Lancet. 1967. 1:1354–1357.
Article25. Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, Novarini A. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002. 346:77–84.
Article26. Na KY, Oh YK, Han JS, Joo KW, Lee JS, Earm JH, Knepper MA, Kim GH. Upregulation of na+ transporter abundances in response to chronic thiazide or loop diuretic treatment in rats. Am J Physiol Renal Physiol. 2003. 284:F133–F143.27. Nijenhuis T, Hoenderop JG, Loffing J, van der Kemp AW, van Os CH, Bindels RJ. Thiazide-induced hypocalciuria is accompanied by a decreased expression of ca2+ transport proteins in kidney. Kidney Int. 2003. 64:555–564.
Article28. Lee CT, Shang S, Lai LW, Yong KC, Lien YH. Effect of thiazide on renal gene expression of apical calcium channels and calbindins. Am J Physiol Renal Physiol. 2004. 287:F1164–F1170.
Article29. Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive ca2+ reabsorption and reduced mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. 2005. 115:1651–1658.
Article30. Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Merillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ. Renal ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking trpv5. J Clin Invest. 2003. 112:1906–1914.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High Dose Vitamin D3 Attenuates the Hypocalciuric Effect of Thiazide in Hypercalciuric Rats
- Expression of Calcium Transporters According to Dietary Sodium in the Distal Tubule of Rat Kidneys
- Immunocytochemical Study of Calcium Binding Protein in the Distal Nephron of Rat Kidney
- Changes of Calbindin -D28k Expression Levels After Transient Ischemic Damage in Rat Cerebellar Purkinje Cells
- Calbindin-D28K Prevents Staurosporin-induced Bax Cleavage and Membrane Permeabilization