J Korean Med Sci.
2008 Aug;23(4):678-684. 10.3346/jkms.2008.23.4.678.
The Interaction of Gabapentin and N(6)-(2-phenylisopropyl)-adenosine R-(-)isomer (R-PIA) on Mechanical Allodynia in Rats with a Spinal Nerve Ligation
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, University of Ulsan, College of Medicine, Seoul Asan Medical Center, Seoul, Korea. jongyeon_park@amc.seoul.kr
- KMID: 1785829
- DOI: http://doi.org/10.3346/jkms.2008.23.4.678
Abstract
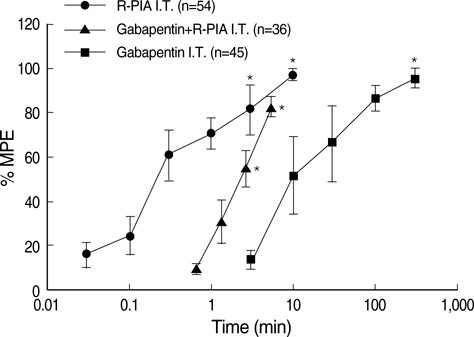
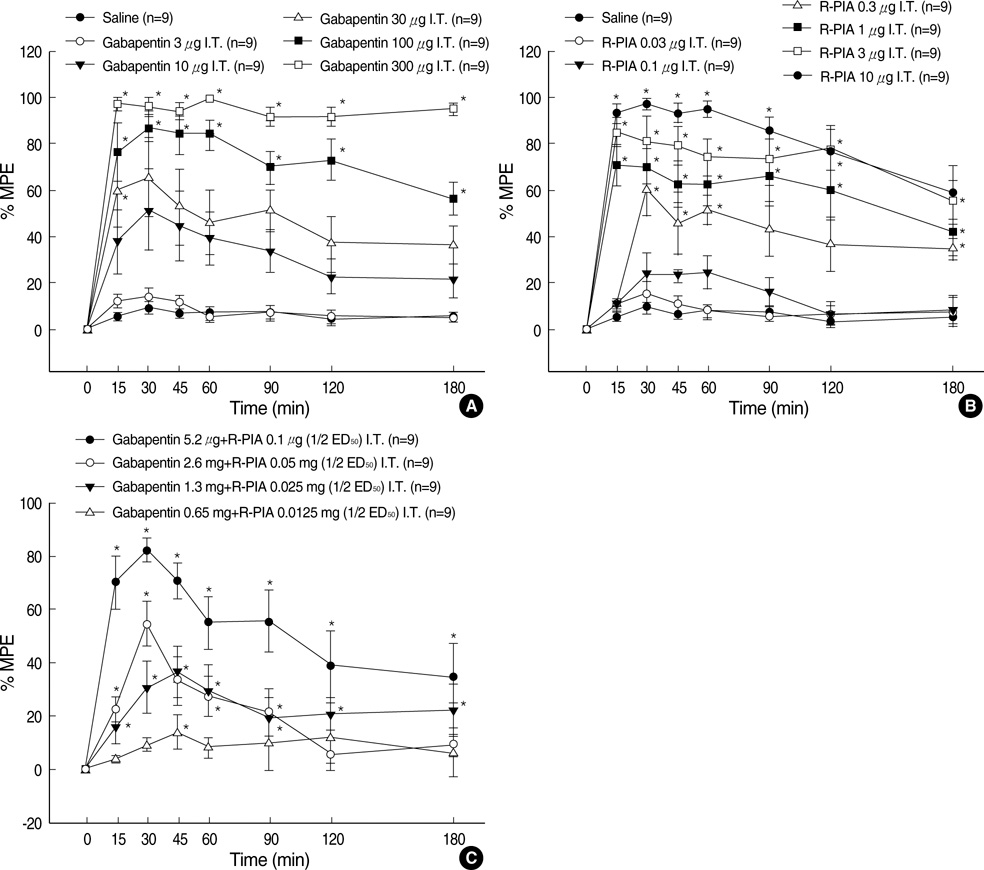
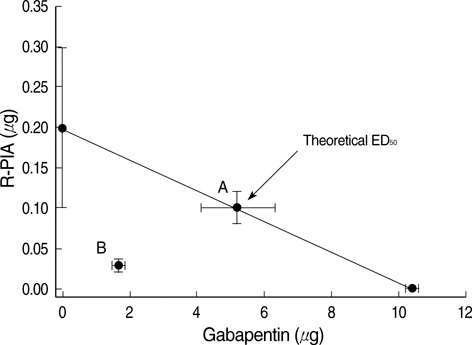
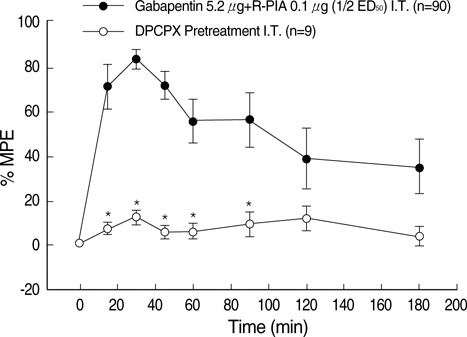
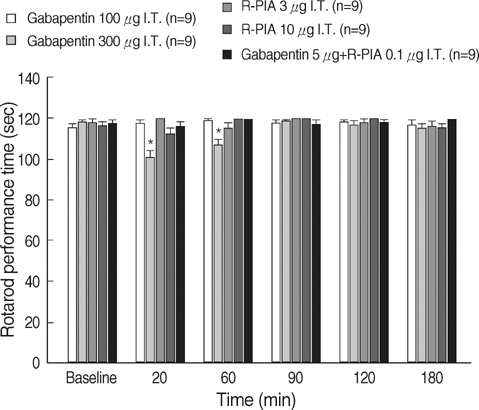
- We examined the antiallodynic interaction between gabapentin and adenosine A1 receptor agonist, N(6)-(2-phenylisopropyl)-adenosine R-(-)isomer (R-PIA), in a rat model of nerve ligation injury. Rats were prepared with ligation of left L5-6 spinal nerves and intrathecal catheter implantation for drug administration. Mechanical allodynia was measured by applying von Frey filaments. Gabapentin and R-PIA were administered to obtain the dose-response curve and the 50% effective dose (ED50). Fractions of ED50s were administered concurrently to establish the ED50 of the drug combination. The drug interaction between gabapentin and R-PIA was analyzed using the isobolographic method. Adenosine A1 receptor antagonist was administered intrathecally to examine the reversal of the antiallodynic effect. Locomotor function changes were evaluated by rotarod testing. Intrathecal gabapentin and R-PIA and their combination produced a dose-dependent antagonism against mechanical allodynia without severe side effects. Intrathecal gabapentin synergistically enhanced the antiallodynic effect of R-PIA when coadministered. There were no significant changes in rotarod performance time, except gabapentin 300 microgram. In the combination group, the maximal antiallodynic effect was reversed by A1 adenosine receptor antagonist. These results suggest that activation of adenosine A1 receptors at the spinal level is required for the synergistic interaction on the mechanical allodynia.
Keyword
MeSH Terms
-
Adenosine/administration & dosage/*analogs & derivatives
Amines/*administration & dosage
Animals
Cyclohexanecarboxylic Acids/*administration & dosage
Dose-Response Relationship, Drug
Drug Synergism
Drug Therapy, Combination
Injections, Spinal
Ligation
Male
Pain/*drug therapy
Rats
Rats, Sprague-Dawley
Receptor, Adenosine A1/drug effects/physiology
Spinal Nerves/*injuries
Xanthines/pharmacology
gamma-Aminobutyric Acid/*administration & dosage
Figure
Cited by 1 articles
-
Antiallodynic and anti-inflammatory effects of intrathecal R-PIA in a rat model of vincristine-induced peripheral neuropathy
Kyungmi Kim, Wonyeong Jeong, In Gu Jun, Jong Yeon Park
Korean J Anesthesiol. 2020;73(5):434-444. doi: 10.4097/kja.19481.
Reference
-
1. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992. 50:355–363.2. Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997. 113:200–206.
Article3. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998. 50:413–492.4. Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998. 347:1–11.
Article5. Sawynok J. Purines in pain management. Curr Opin CPNS Invest Drugs. 1999. 1:27–38.6. Klotz KN. Adenosine receptors and their ligands. Naunyn Schmiedebergs Arch Pharmacol. 2000. 362:382–391.
Article7. Lee YW, Yaksh TL. Pharmacology of the spinal adenosine receptor which mediates the antiallodynic action of intrathecal adenosine agonists. J Pharmacol Exp Ther. 1996. 277:1642–1648.8. Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, Myers RR. Injury type-Specific calcium channel α2δ-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002. 303:1199–1205.9. Field MJ, Oles RJ, Lewis AS, McCleary S, Hughes J, Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol. 1997. 121:1513–1522.
Article10. Eckhardt K, Ammon S, Hofmann U, Riebe A, Gugeler N, Mikus G. Gabapentin enhances the analgesic effect of morphine in healthy volunteers. Anesth Analg. 2000. 91:185–191.
Article11. Matthews EA, Dickenson AH. A combination of gabapentin and morphine mediates enhanced inhibitory effects on dorsal horn neuronal responses in a rat model of neuropathy. Anesthesiology. 2002. 96:633–640.
Article12. Gilron I, Biederman J, Jhamandas K, Hong M. Gabapentin blocks and reverses antinociceptive morphine tolerance in the rat paw-pressure and tail-flick tests. Anesthesiology. 2003. 98:1288–1292.
Article13. Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003. 60:1524–1534.14. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976. 17:1031–1036.
Article15. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994. 53:55–63.
Article16. Tallarida RJ, Porreca F, Cowan A. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life Sci. 1989. 45:947–961.
Article17. Cui JG, Sollevi A, Linderoth B, Meyerson BA. Adenosine receptor activation suppresses tactile hypersensitivity and potentiates spinal cord stimulation in mononeuropathic rats. Neurosci Lett. 1997. 223:173–176.
Article18. von Heijne M, Hao JX, Sollevi A, Xu XJ, Wiesenfeld-Hallin Z. Marked enhancement of anti-allodynic effect by combined intrathecal administration of the adenosine A1-receptor agonist R-phenylisopropyladenosine and morphine in a rat model of central pain. Acta Anaesthesiol Scand. 2000. 44:665–671.
Article19. Sosnowski M, Yaksh TL. Role of spinal adenosine receptors in modulating the hyperesthesia produced by spinal glycine receptor antagonism. Anesth Analg. 1989. 69:587–592.
Article20. Choca JI, Proudfit HK, Green RD. Identification of A1 and A2 adenosine receptors in the rat spinal cord. J Pharmacol Exp Ther. 1987. 242:905–910.21. Bantel C, Tobin JR, Li X, Childers SR, Chen SR, Eisenach JC. Intrathecal adenosine following spinal nerve ligation in rat: short residence time in cerebrospinal fluid and no change in A(1) receptor binding. Anesthesiology. 2002. 96:103–108.22. Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, Dooley DJ, Boden P, Singh L. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998. 29:233–249.
Article23. Yoon MH, Yaksh TL. The effect of intrathecal gabapentin on pain behavior and hemodynamics on the formalin test in the rat. Anesth Analg. 1999. 89:434–439.
Article24. Matthews EA, Dickenson AH. A combination of gabapentin and morphine mediates enhanced inhibitory effects on dorsal horn neuronal responses in a rat model of neuropathy. Anesthesiology. 2002. 96:633–640.
Article25. Chapman V, Suzuki R, Chamarette HL, Rygh LJ, Dickenson AH. Effects of systemic carbamazepine and gabapentin on spinal neuronal responses in spinal nerve ligated rats. Pain. 1998. 75:261–272.
Article26. Petroff OA, Hyder F, Rothman DL, Mattson RH. Effects of gabapentin on brain GABA, homocarnosine, and pyrrolidinone in epilepsy patients. Epilepsia. 2000. 41:675–680.
Article27. Su TZ, Lunney E, Campbell G, Oxender DL. Transport of gabapentin, a gamma-amino acid drug, by system L alpha-amino acid transporters: a comparative study in astrocytes, synaptosomes, and CHO cells. J Neurochem. 1995. 64:2125–2131.28. Shimoyama M, Shimoyama N, Hori Y. Gabapentin affects glutamatergic excitatory neurotransmission in the rat dorsal horn. Pain. 2000. 85:405–414.
Article29. Maneuf YP, McKnight AT. Block by gabapentin of the facilitation of glutamate release from rat trigeminal nucleus following activation of protein kinase C or adenylyl cyclase. Br J Pharmacol. 2001. 134:237–240.
Article30. Leem JW, Choi EJ, Park ES, Paik KS. N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptor antagonists differentially suppress dorsal horn neuron responses to mechanical stimuli in rats with peripheral nerve injury. Neurosci Lett. 1996. 211:37–40.
Article31. Fink K, Meder W, Dooley DJ, Gothert M. Inhibition of neuronal Ca2+ influx by gabapentin and subsequent reduction of neurotransmitter release from rat neocortical slices. Br J Pharmacol. 2000. 130:900–906.32. Hwang JH, Hwang GS, Cho SK, Han SM. Morphine can enhance the antiallodynic effect of intrathecal R-PIA in rats with nerve ligation injury. Anesth Analg. 2005. 100:461–468.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Mechanical Antiallodynic Effect of intrathecal Morphine and R-Phenylisopropyl-Adenosine in Rats with Spinal Nerve Ligation
- Antiallodynic and anti-inflammatory effects of intrathecal R-PIA in a rat model of vincristine-induced peripheral neuropathy
- The Mechanism of R-PIA Induced Mechanical Antiallodynia in a Peripheral Neuropathic Rat
- The Interaction of Morphine and Adenosine Receptor Agonists on Antiallodynia in Rats with a Nerve Ligation Injury
- The Effect of ATP-sensitive Potassium Channel on R-PIA Induced Mechanical Antiallodynia in a Peripheral Neuropathic Rat