J Korean Med Sci.
2007 Sep;22(Suppl):S73-S78. 10.3346/jkms.2007.22.S.S73.
Change of the Expression of Human Telomerase Reverse Transcriptase mRNA and Human Telomerase RNA after Cisplatin and 5-Fluorouracil Exposure in Head and Neck Squamous Cell Carcinoma Cell Lines
- Affiliations
-
- 1Department of Otolaryngology, College of Medicine, Pusan National University, Busan, Korea. gohek@pusan.ac.kr
- 2Department of Biochemistry, College of Medicine, Pusan National University, Busan, Korea.
- 3Medical Research Institute, Pusan National University Hospital, Busan, Korea.
- KMID: 1785792
- DOI: http://doi.org/10.3346/jkms.2007.22.S.S73
Abstract
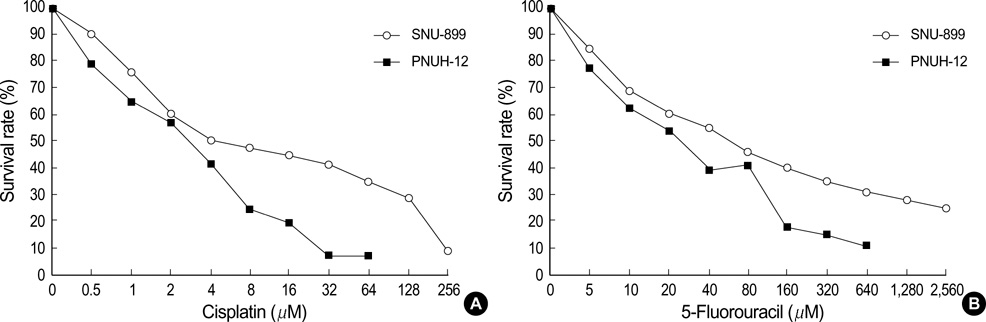
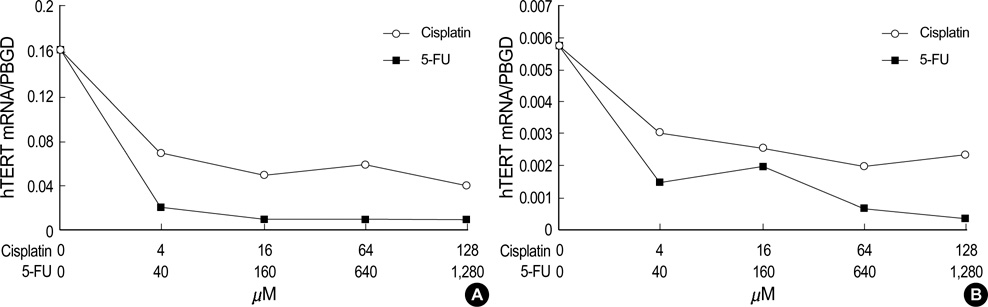
- Telomerase activity appears to be associated with cell immortalization and malignant progression. Understanding how telomerase activity is regulated in vivo is important not only for understanding the molecular biology of telomerase but also for the potential clinical application of anticancer drugs. This study evaluated telomerase activity and quantified the expression of human telomerase reverse transcriptase (hTERT) mRNA and human telomerase RNA (hTR) using a real-time reverse transcriptase-polymerase chain reaction (RT-PCR) method before and after the exposure of cisplatin and 5-fluorouracil (5-FU) in two head and neck squamous cell carcinoma (HNSCC) cell lines. Two human HNSCC cell lines (PNUH-12 and SNU-899) were studied. Cell cytotoxicity, the change of telomerase activity, and hTERT mRNA and hTR expression by 5-FU and cisplatin exposure were assessed by MTT assay, TRAP assay, and real-time RT-PCR, respectively. In two cell lines, after cisplatin exposure, the telomerase activity and hTERT mRNA expression decreased, but hTR expression in- creased according to the concentration of drug. However, in both cell lines, the telomerase activity and hTR did not show any significant change after 5-FU treatment, but the expression of hTERT mRNA decreased. These results suggest that there may be other important regulating mechanism except hTERT mRNA as the regulation factor of telomerase activity in HNSCC cell lines.
MeSH Terms
-
Antineoplastic Agents/pharmacology
Base Sequence
Carcinoma, Squamous Cell/*drug therapy/*enzymology/genetics
Cell Line, Tumor
Cisplatin/*pharmacology
DNA Primers/genetics
Fluorouracil/*pharmacology
Gene Expression/drug effects
Head and Neck Neoplasms/*drug therapy/*enzymology/genetics
Humans
RNA, Messenger/*genetics/*metabolism
RNA, Neoplasm/*genetics/*metabolism
Reverse Transcriptase Polymerase Chain Reaction
Telomerase/*genetics/*metabolism
Figure
Cited by 1 articles
-
Growth Inhibitory Effect of Palatine Tonsil-derived Mesenchymal Stem Cells on Head and Neck Squamous Cell Carcinoma Cells
Yun-Sung Lim, Jin-Choon Lee, Yoon Se Lee, Byung-Joo Lee, Soo-Geun Wang
Clin Exp Otorhinolaryngol. 2012;5(2):86-93. doi: 10.3342/ceo.2012.5.2.86.
Reference
-
1. Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994. 266:2011–2015.
Article2. Lee BJ, Wang SG, Choi JS, Lee JC, Goh EK, Kim MG. The prognostic value of telomerase expression in peripheral blood mononuclear cells of head and neck cancer patients. Am J Clin Oncol. 2006. 29:163–167.
Article3. Liao CT, Tung-Chieh Chang J, Wang HM, Chen IH, Lin CY, Chen TM, Hsieh LL, Cheng AJ. Telomerase as an independent prognostic factor in head and neck squamous cell carcinoma. Head Neck. 2004. 24:504–512.
Article4. Yu HP, Xu SQ, Lu WH, Li YY, Li F, Wang XL, Su YH. Telomerase activity and expression of telomerase genes in squamous dysplasia and squamous cell carcinoma of the esophagus. J Surg Oncol. 2004. 86:99–104.
Article5. Shibuya K, Fujisawa T, Hoshino H, Baba M, Saitoh Y, Iizasa T, Sekine Y, Suzuki M, Hiroshima K, Ohwada H. Increased telomerase activity and elevated hTERT mRNA expression during multistage carcinogenesis of squamous cell carcinoma of the lung. Cancer. 2001. 92:849–855.
Article6. Ducrest AL, Szutorisz H, Lingner J, Nabholz M. Regulation of the human telomerase reverse transcriptase gene. Oncogene. 2002. 21:541–552.
Article7. Wisman GB, Knol AJ, Helder MN, Krans M, de Vries EG, Hollema H, de Jong S, van der Zee AG. Telomerase in relation to clinicopathologic prognostic factors and survival in cervical cancer. Int J Cancer. 2001. 91:658–664.
Article8. Kanaya T, Kyo S, Takakura M, Ito H, Namiki M, Inoue M. hTERT is a critical determinant of telomerase activity in renal-cell carcinoma. Int J Cancer. 1998. 78:539–543.
Article9. Majem M, Mesia R, Manos M, Gomez J, Galiana R, Cardenal F, Juan A, Montes A, Perez FJ, Nogues J, Llluch JR. Does induction chemotherapy still have a role in larynx preservation strategies? The experience of Institut Catala d'Oncologia in stage III larynx carcinoma. Laryngoscope. 2006. 116:1651–1656.
Article10. Ku JL, Kim WH, Lee JH, Park HS, Kim KH, Sung MW, Park JG. Establishment and characterization of human laryngeal squamous cell carcinoma cell lines. Laryngoscope. 1999. 109:976–982.
Article11. Roh HJ, Goh EK, Wang SG, Chon KM, Kim YS, Han JY. Establishment and Characterization of a Novel Cell Line (PNUH-12) Derived from a Human Squamous Cell Carcinoma of the Hypopharynx. Korean J Otolaryngol. 1999. 42:72–81.12. Akiyama M, Horiguchi-Yamada J, Saito S, Hoshi Y, Yamada O, Mizoguchi H, Yamada H. Cytostatic concentrations of anticancer agents do not affect telomerase activity of leukaemic cells in vitro. Eur J Cancer. 1999. 35:309–315.
Article13. Cressey TR, Tilby MJ, Newell DR. Decreased telomerase activity is not a reliable indicatior of chemosensitivity in testicular cancer cell lines. Eur J Cancer. 2002. 38:586–593.14. Lee BJ, Chon KM, Kim YS, An WG, Roh HJ, Goh EK, Wang SG. The effects of cisplatin, 5-fluorouracil, and radiation on cell cycle regulation and apoptosis in the hypopharyngeal carcinoma cell line. Chemotherapy. 2005. 51:103–110.15. Ku WC, Cheng AJ, Wang TC. Inhibition of telomerase activity by PKC inhibitors in human nasopharyngeal cancer cells in culture. Biochem Biophys Res Commun. 1997. 241:730–736.
Article16. Lin Z, Lim S, Viani MA, Sapp M, Lim MS. Down-regulation of telomerase activity in malignant lymphomas by radiation and chemotherapeutic agents. Am J Pathol. 2001. 159:711–719.
Article17. Bieche I, Nogues C, Paradis V, Olivi M, Bedossa P, Lidereau R, Vidaud M. Quantitation of hTERT gene expression in sporadic breast tumors with a real-time reverse transcription-polymerase chain reaction assay. Clin Cancer Res. 2000. 6:452–459.18. Rohde V, Sattler HP, Bund T, Bonkhoff H, Fixemer T, Bachmann C, Lensch R, Unteregger G, Stoeckle M, Wullich B. Expression of the human telomerase reverse transcriptase is not related to telomerase activity in normal and malignant renal tissue. Clin Cancer Res. 2000. 6:4803–4809.19. Bustin SA, Mueller R. Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis. Clin Sci (Lond). 2005. 109:365–379.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Change of Telomerase Activity by Cisplatin and 5-FU in Head and Neck Cancer Cell Lines
- Telomerase Activity and Expression of hTR and TERT in Human Soft Tissue Sarcomas
- The Expression of Telomerase Reverse Transcriptase Protein is an Independent Prognostic Marker in Early Stage Non-Small Cell Lung Carcinomas
- The Inhibition of Human Telomerase Reverse Transcriptase Expression by Peroxiredoxin I and c-Myc in Prostatic Cancer Cells
- Mouse models for telomere and telomerase biology