J Korean Med Sci.
2004 Feb;19(1):55-61. 10.3346/jkms.2004.19.1.55.
Effects of Propofol on Endotoxin-Induced Acute Lung Injury in Rabbit
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chonnam National University, Medical School, Gwangju, Korea. shkwak@chonnam.ac.kr
- 2Department of Legal Medicine, Chonnam National University, Medical School, Gwangju, Korea.
- KMID: 1785694
- DOI: http://doi.org/10.3346/jkms.2004.19.1.55
Abstract
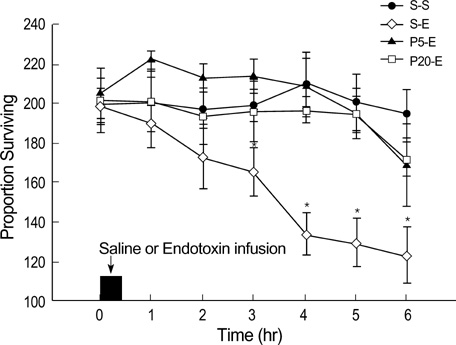
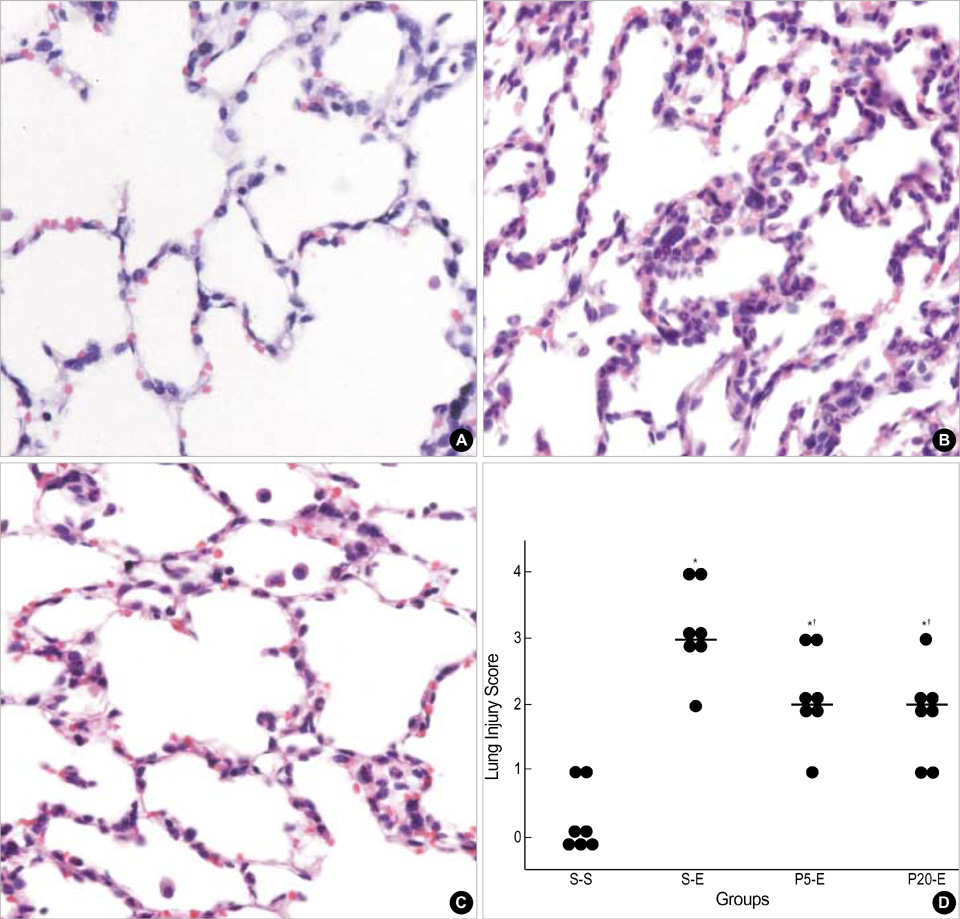
- This study was undertaken to clarify the effects of propofol on endotoxin-induced acute lung injury. Rabbits were randomly assigned to one of four groups. Each group received intravenous infusion of saline only, saline and Escherichia coli endotoxin, propofol (1 mg/kg bolus, then 5 mg/kg/hr) and endotoxin, or propofol (4 mg/kg bolus, then 20 mg/kg/hr) and endotoxin respectively. Infusion of saline or propofol was started 0.5 hr before the infusion of saline or endotoxin, and continued for 6 hr thereafter. The lungs of rabbits were ventilated with 40% oxygen. Mean blood pressure, heart rate, arterial oxygen tension (PaO2), and peripheral blood leukocyte and platelet count were recorded. The wet/dry (W/D) weight ratio of lung and lung injury score were measured, and analysis of bronchoalveolar lavage fluid (BALF) was done. Endotoxin decreased PaO2, and peripheral blood leukocyte and platelet count. And it increased W/D ratio of lung, lung injury score and leukocyte count, percentage of PMN cells, concentration of albumin, thromboxane B2 and IL-8 in BALF. Propofol attenuated all these changes except the leukocyte count in peripheral blood. In conclusion, propofol attenuated endotoxin-induced acute lung injury in rabbits mainly by inhibiting neutrophil and IL-8 responses, which may play a central role in sepsis-related lung injury.
MeSH Terms
-
Acute Disease
Albumins/metabolism
Anesthetics, Intravenous/pharmacology
Animals
Blood Platelets/metabolism
Blood Pressure
Bronchoalveolar Lavage Fluid
Endotoxins/*metabolism
Escherichia coli/metabolism
Free Radical Scavengers/pharmacology
Interleukin-8/metabolism
Leukocytes/metabolism
Lung/*injuries/pathology
Male
Organ Weight
Oxygen/metabolism
Propofol/*pharmacology
Rabbits
Sepsis
Sodium Chloride/pharmacology
Thromboxane B2/metabolism
Time Factors
Figure
Cited by 2 articles
-
Effects of Propofol and Gabexate Mesilate (Foy) on Endotoxin-induced Acute Lung Injury in Rabbit
Sung-Su Chung, Hong-Beom Bae, Seok-Jae Kim, Cheol-Won Jeong, Hye-Jin Chung, Mai Li, Sang-Hyun Kwak
Chonnam Med J. 2008;44(3):169-176. doi: 10.4068/cmj.2008.44.3.169.The volatile anesthetic sevoflurane attenuates ventilator-induced lung injury through inhibition of ERK1/2 and Akt signal transduction
Sang-Hun Kim, Mei Li, Tae-Hee Pyeon, Keum-Young So, Sang-Hyun Kwak
Korean J Anesthesiol. 2015;68(1):62-69. doi: 10.4097/kjae.2015.68.1.62.
Reference
-
1. Kollef MH, Schuster DP. The acute respiratory distress syndrome. N Engl J Med. 1995. 332:27–37.
Article2. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994. 149:818–824.
Article3. Sloane PJ, Gee MH, Gottlieb JE, Albertine KH, Peters SP, Burns JR, Machiedo G, Fish JE. A multicenter registry of patients with acute respiratory distress syndrome. Physiology and outcome. Am Rev Respir Dis. 1992. 146:419–426.
Article4. Vasilyev S, Schaap RN, Mortensen JD. Hospital survival rates of patients with acute respiratory failure in modern respiratory intensive care units. An international, multicenter, prospective survey. Chest. 1995. 107:1083–1088.5. Knaus WA, Sun X, Hakim RB, Wagner DP. Evaluation of definitions for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994. 150:311–317.
Article6. Repine JE. Scientific perspective on adult respiratory distress syndrome. Lancet. 1992. 339:466–469.7. Chollet-Martin S, Jourdain B, Gibert C, Elbim C, Chastre J, Gougerot-Pocidalo MA. Interactions between neutrophils and cytokines in blood and alveolar spaces during ARDS. Am J Respir Crit Care Med. 1996. 154:594–601.
Article8. Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996. 154:602–611.
Article9. Suter PM, Suter S, Girardin E, Roux-Lombard P, Grau GE, Dayer JM. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992. 145:1016–1022.
Article10. Jacobs RF, Tabor DR, Burks AW, Campbell GD. Elevated interleukin-1 release by human alveolar macrophages during the adult respiratory distress syndrome. Am Rev Respir Dis. 1989. 140:1686–1692.11. Okumura Y, Inoue H, Fujiyama Y, Bamba T. Effects of serine protease inhibitors on accumulation of polymorphonuclear leukocytes in the lung induced by acute pancreatitis in rats. J Gastroenterol. 1995. 30:379–386.
Article12. McCord JM, Gao B, Leff J, Flores SC. Neutrophil-generated free radicals: possible mechanisms of injury in adult respiratory distress syndrome. Environ Health Perspect. 1994. 102:Suppl 10. 57–60.
Article13. Murphy PG, Bennett JR, Myers DS, Davies MJ, Jones JG. The effect of propofol anaesthesia on free radical-induced lipid peroxidation in rat liver microsomes. Eur J Anaesthesiol. 1993. 10:261–266.14. Mathy-Hartert M, Deby-Dupont G, Hans P, Deby C, Lamy M. Protective activity of propofol, Diprivan and intralipid against active oxygen species. Mediators Inflamm. 1998. 7:327–333.15. Murphy PG, Myers DS, Davies MJ, Webster NR, Jones JG. The antioxidant potential of propofol (2,6-diisopropylphenol). Br J Anaesth. 1992. 68:613–618.16. Heine J, Leuwer M, Scheinichen D, Arseniev L, Jaeger K, Piepenbrock S. Flow cytometry evaluation of the in vitro influence of four i.v. anaesthetics on respiratory burst of neutrophils. Br J Anaesth. 1996. 77:387–392.
Article17. Mikawa K, Akamatsu H, Nishina K, Shiga M, Maekawa N, Obara H, Niwa Y. Propofol inhibits human neutrophil functions. Anesth Analg. 1998. 87:695–700.
Article18. Aoki H, Mizobe T, Nozuchi S, Hiramatsu N. In vivo and in vitro studies of the inhibitory effect of propofol on human platelet aggregation. Anesthesiology. 1998. 88:362–370.
Article19. Galley HF, Dubbels AM, Webster NR. The effect of midazolam and propofol on interleukin-8 from human polymorphonuclear leukocytes. Anesth Analg. 1998. 86:1289–1293.
Article20. Perez-Martinez A, Gonzalvez-Pinera J, Marco-Macian A, Carpintero-Moreno F, Moya-Marchante M. [Propofol in continuous perfusion as anesthetic in experimental surgery in the rabbit]. Rev Esp Anestesiol Reanim. 1995. 42:253–256.21. Ichinohe T, Aida H, Kaneko Y. Interaction of nitrous oxide and propofol to reduce hypertensive response to stimulation. Can J Anaesth. 2000. 47:699–704.
Article22. Matsukawa A, Yoshimura T, Miyamoto K, Ohkawara S, Yoshinaga M. Analysis of the inflammatory cytokine network among TNF alpha, IL-1 beta, IL-1 receptor antagonist, and IL-8 in LPS-induced rabbit arthritis. Lab Invest. 1997. 76:629–638.23. Demling RH. Adult respiratory distress syndrome: current concepts. New Horiz. 1993. 1:388–401.24. Touqui L, Arbibe L. A role for phospholipase A2 in ARDS pathogenesis. Mol Med Today. 1999. 5:244–249.
Article25. Brigham KL, Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis. 1986. 133:913–927.26. Bernard C, Tedgui A. Cytokine network and the vessel wall. Insights into septic shock pathogenesis. Eur Cytokine Netw. 1992. 3:19–33.27. Lamy M, Deby-Dupont G, Deby C, Pincemail J, Duchateau J, Braun M, Damas P, Roth M. Artigs A, Lemaire F, Suter PM, Zapol WM, editors. Measurements of mediator cascade during adult respiratory distress syndrome. Adult Respiratory Distress Syndrome. 1992. New York: Churchill Livingstone;71–88.28. Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987. 237:1171–1176.
Article29. Spragg RG. Artigs A, Lemaire F, Suter PM, Zapol WM, editors. Platelets and acute lung injury. Adult Respiratory Distress Syndrome. 1992. New York: Churchill Livingstone;89–94.30. Christman BW, Lefferts PL, Blair IA, Snapper JR. Effect of platelet-activating factor receptor antagonism on endotoxin-induced lung dysfunction in awake sheep. Am Rev Respir Dis. 1990. 142:1272–1278.
Article31. Glauser FL, Fairman RP. The uncertain role of the neutrophil in increased permeability pulmonary edema. Chest. 1985. 88:601–607.
Article32. Basu S, Mutschler DK, Larsson AO, Kiiski R, Nordgren A, Eriksson MB. Propofol (Diprivan-EDTA) counteracts oxidative injury and deterioration of the arterial oxygen tension during experimental septic shock. Resuscitation. 2001. 50:341–348.
Article33. Inada T, Taniuchi S, Shingu K, Kobayashi Y, Fujisawa J, Nakao S. Propofol depressed neutrophil hydrogen peroxide production more than midazolam, whereas adhesion molecule expression was minimally affected by both anesthetics in rats with abdominal sepsis. Anesth Analg. 2001. 92:437–441.
Article34. Crozier TA, Muller JE, Quittkat D, Sydow M, Wuttke W, Kettler D. Effect of anaesthesia on the cytokine responses to abdominal surgery. Br J Anaesth. 1994. 72:280–285.
Article35. Taniguchi T, Yamamoto K, Ohmoto N, Ohta K, Kobayashi T. Effects of propofol on hemodynamic and inflammatory responses to endotoxemia in rats. Crit Care Med. 2000. 28:1101–1106.
Article36. Hirakata H, Nakamura K, Yokubol B, Toda H, Hatano Y, Urabe N, Mori K. Propofol has both enhancing and suppressing effects on human platelet aggregation in vitro. Anesthesiology. 1999. 91:1361–1369.
Article37. De La Cruz JP, Zanca A, Carmona JA, de la Cuesta FS. The effect of propofol on oxidative stress in platelets from surgical patients. Anesth Analg. 1999. 89:1050–1055.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Propofol and Gabexate Mesilate (Foy) on Endotoxin-induced Acute Lung Injury in Rabbit
- Effects of Gabexate Mesilate (Foy(R)) on Endotoxin-Induced Acute Lung Injury in Rabbit
- Inhibition of Phospholipase A2 Ameliorates the Acute Lung Injury Induced by E Coli Endotoxin via Reduced Production of Oxygen Free Radicals in the Lung
- The effect of surfactant therapy for acute lung injury induced by intratracheal endotoxin instillation in rats examination of the lung
- The role of hydroxyl radical in the pathogenetic mechanism of endotoxin-induced acute lung injury in rats