J Vet Sci.
2014 Jun;15(2):209-216. 10.4142/jvs.2014.15.2.209.
Production and immunogenicity of chimeric virus-like particles containing the spike glycoprotein of infectious bronchitis virus
- Affiliations
-
- 1State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University, Guangzhou 510006, China. caoych@mail.sysu.edu.cn
- KMID: 1784641
- DOI: http://doi.org/10.4142/jvs.2014.15.2.209
Abstract
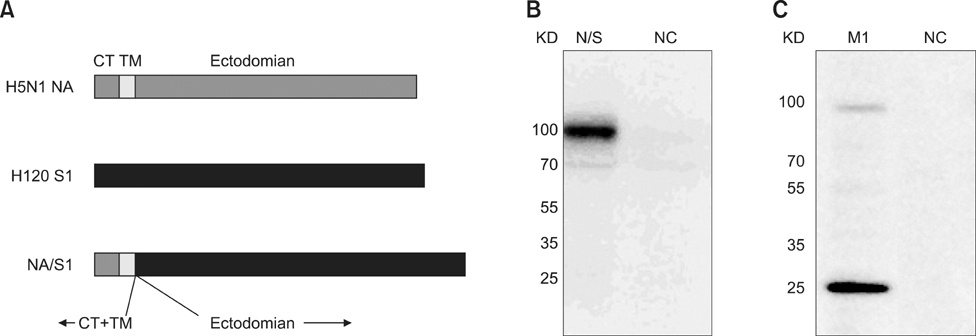
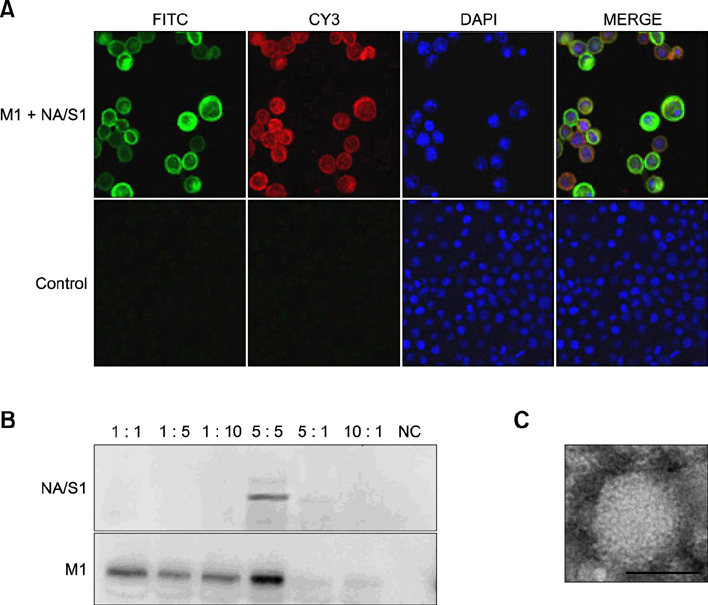
- Infectious bronchitis virus (IBV) poses a severe threat to the poultry industry and causes heavy economic losses worldwide. Vaccination is the most effective method of preventing infection and controlling the spread of IBV, but currently available inactivated and attenuated virus vaccines have some disadvantages. We developed a chimeric virus-like particle (VLP)-based candidate vaccine for IBV protection. The chimeric VLP was composed of matrix 1 protein from avian influenza H5N1 virus and a fusion protein neuraminidase (NA)/spike 1 (S1) that was generated by fusing IBV S1 protein to the cytoplasmic and transmembrane domains of NA protein of avian influenza H5N1 virus. The chimeric VLPs elicited significantly higher S1-specific antibody responses in intramuscularly immunized mice and chickens than inactivated IBV viruses. Furthermore, the chimeric VLPs induced significantly higher neutralization antibody levels than inactivated H120 virus in SPF chickens. Finally, the chimeric VLPs induced significantly higher IL-4 production in mice. These results demonstrate that chimeric VLPs have the potential for use in vaccines against IBV infection.
MeSH Terms
-
Animals
Antibodies, Viral/blood
*Chickens
Chimera/genetics/immunology
Coronavirus Infections/prevention & control/*veterinary/virology
Female
*Immunity, Innate
Infectious bronchitis virus/genetics/*immunology
Influenza A Virus, H5N1 Subtype/genetics/immunology
Injections, Intramuscular/veterinary
Mice
Mice, Inbred BALB C
Neuraminidase/genetics
Poultry Diseases/*prevention & control/virology
Recombinant Fusion Proteins/genetics/immunology
Spike Glycoprotein, Coronavirus/genetics/*immunology
Vaccines, Synthetic/administration & dosage/genetics/immunology
Vaccines, Virus-Like Particle/administration & dosage/genetics/*immunology
Viral Proteins/genetics
Antibodies, Viral
Recombinant Fusion Proteins
Neuraminidase
Spike Glycoprotein, Coronavirus
Vaccines, Synthetic
Vaccines, Virus-Like Particle
Viral Proteins
Figure
Reference
-
1. Ammayappan A, Upadhyay C, Gelb J Jr, Vakharia VN. Complete genomic sequence analysis of infectious bronchitis virus Ark DPI strain and its evolution by recombination. Virol J. 2008; 5:157.
Article2. Arndt AL, Larson BJ, Hogue BG. A conserved domain in the coronavirus membrane protein tail is important for virus assembly. J Virol. 2010; 84:11418–11428.
Article3. Barman S, Adhikary L, Chakrabarti AK, Bernas C, Kawaoka Y, Nayak DP. Role of transmembrane domain and cytoplasmic tail amino acid sequences of influenza a virus neuraminidase in raft association and virus budding. J Virol. 2004; 78:5258–5269.
Article4. Bijlenga G, Cook JKA, Gelb J Jr, de Wit JJ. Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine: a review. Avian Pathol. 2004; 33:550–557.
Article5. Boltz DA, Nakai M, Bahra JM. Avian infectious bronchitis virus: a possible cause of reduced fertility in the rooster. Avian Dis. 2004; 48:909–915.
Article6. Boudko SP, Londer YY, Letarov AV, Sernova NV, Engel J, Mesyanzhinov VV. Domain organization, folding and stability of bacteriophage T4 fibritin, a segmented coiled-coil protein. Eur J Biochem. 2002; 269:833–841.
Article7. Casais R, Dove B, Cavanagh D, Britton P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J Virol. 2003; 77:9084–9089.
Article8. Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet Res. 2007; 38:281–297.
Article9. Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003; 32:567–582.
Article10. Cavanagh D, Casais R, Armesto M, Hodgson T, Izadkhasti S, Davies M, Lin F, Tarpey I, Britton P. Manipulation of the infectious bronchitis coronavirus genome for vaccine development and analysis of the accessory proteins. Vaccine. 2007; 25:5558–5562.
Article11. Cavanagh D, Davis PJ. Coronavirus IBV: removal of spike glycopolypeptide S1 by urea abolishes infectivity and haemagglutination but not attachment to cells. J Gen Virol. 1986; 67:1443–1448.
Article12. Cavanagh D, Davis PJ, Darbyshire JH, Peters RW. Coronavirus IBV: virus retaining spike glycopolypeptide S2 but not S1 is unable to induce virus-neutralizing or haemagglutination-inhibiting antibody, or induce chicken tracheal protection. J Gen Virol. 1986; 67:1435–1442.
Article13. Cavanagh D, Picault JP, Gough R, Hess M, Mawditt K, Britton P. Variation in the spike protein of the 793/B type of infectious bronchitis virus, in the field and during alternate passage in chickens and embryonated eggs. Avian Pathol. 2005; 34:20–25.
Article14. Cox MM. Progress on baculovirus-derived influenza vaccines. Curr Opin Mol Ther. 2008; 10:56–61.15. Galarza JM, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005; 18:244–251.
Article16. Gelb J Jr, Weisman Y, Ladman BS, Meir R. S1 gene characteristics and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996 to 2000). Avian Pathol. 2005; 34:194–203.
Article17. de Haan CA, Kuo L, Masters PS, Vennema H, Rottier PJ. Coronavirus particle assembly: primary structure requirements of the membrane protein. J Virol. 1998; 72:6838–6850.
Article18. Haynes JR. Influenza virus-like particle vaccines. Expert Rev Vaccines. 2009; 8:435–445.
Article19. Kant A, Koch G, van Roozelaar DJ, Kusters JG, Poelwijk FA, van der Zeijst BA. Location of antigenic sites defined by neutralizing monoclonal antibodies on the S1 avian infectious bronchitis virus glycopolypeptide. J Gen Virol. 1992; 73:591–596.
Article20. Ladman BS, Loupos AB, Gelb J Jr. Infectious bronchitis virus S1 gene sequence comparison is a better predictor of challenge of immunity in chickens than serotyping by virus neutralization. Avian Pathol. 2006; 35:127–133.
Article21. Latham T, Galarza JM. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J Virol. 2001; 75:6154–6165.
Article22. Liu G, Lv L, Yin L, Li X, Luo D, Liu K, Xue C, Cao Y. Assembly and immunogenicity of coronavirus-like particles carrying infectious bronchitis virus M and S proteins. Vaccine. 2013; 31:5524–5530.
Article23. Lokugamage KG, Yoshikawa-lwata N, Ito N, Watts DM, Wyde PR, Wang N, Newman P, Tseng CTK, Peters CJ, Makino S. Chimeric coronavirus-like particles carrying severe acute respiratory syndrome coronavirus (SCoV) S protein protects mice against challenge with SCoV. Vaccine. 2008; 26:797–808.
Article24. Mahmood K, Bright RA, Mytle N, Carter DM, Crevar CJ, Achenbach JE, Heaton PM, Tumpey TM, Ross TM. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine. 2008; 26:5393–5399.
Article25. McKinley ET, Hilt DA, Jackwood MW. Avian coronavirus infectious bronchitis attenuated live vaccines undergo selection of subpopulations and mutations following vaccination. Vaccine. 2008; 26:1274–1284.
Article26. Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Heaton PM, Fraire AE, Morrison TG. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J Virol. 2010; 84:1110–1123.
Article27. Noad R, Roy P. Virus-like particles as immunogens. Trends Microbiol. 2003; 11:438–444.
Article28. Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005; 23:5751–5759.
Article29. Raj GD, Jones RC. Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian Pathol. 1997; 26:677–706.
Article30. Roldão A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010; 9:1149–1176.
Article31. Siu YL, Teoh KT, Lo J, Chen CM, Kien F, Escriou N, Tsao SW, Nicholls JM, Altmeyer R, Peiris JS, Bruzzone R, Nal B. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J Virol. 2008; 82:11318–11330.
Article32. Sjaak de Wit JJ, Cook JK, van der Heijden HM. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011; 40:223–235.
Article33. Tao P, Luo M, Zhu D, Qu S, Ynag Z, Gao M, Guo D, Pan Z. Virus-like particle vaccine comprised of the HA, NA, and M1 proteins of an avian isolated H5N1 influenza virus induces protective immunity against homologous and heterologous strains in mice. Viral Immunol. 2009; 22:273–281.
Article34. Wang L, Junker D, Hock L, Ebiary E, Collisson EW. Evolutionary implications of genetic variations in the S1 gene of infectious bronchitis virus. Virus Res. 1994; 34:327–338.
Article35. Weber J, Cheinsong-Popov R, Callow D, Adams S, Patou G, Hodgkin K, Martin S, Gotch F, Kingsman A. Immunogenicity of the yeast recombinant p17/p24:Ty virus-like particles (p24-VLP) in healthy volunteers. Vaccine. 1995; 13:831–834.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of a mimotope of an infectious bronchitis virus S1 protein
- Development of an attenuated vaccine strain from a korean respiratory type infectious bronchitis virus
- Progress and hurdles in the development of influenza virus-like particle vaccines for veterinary use
- Correlations in the results of virus neutralization test, hemagglutination inhibition test, and enzyme-linked immunosorbent assay to determine infectious bronchitis virus vaccine potency
- Immunosuppressive effect of Cryptosporidium baileyi infection on vaccination against avian infectious bronchitis in chicks