J Periodontal Implant Sci.
2011 Oct;41(5):218-226. 10.5051/jpis.2011.41.5.218.
The evaluation of the correlation between histomorphometric analysis and micro-computed tomography analysis in AdBMP-2 induced bone regeneration in rat calvarial defects
- Affiliations
-
- 1Department of Periodontology, Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. yjseol@snu.ac.kr
- 2Mediflower Clinic, Seoul, Korea.
- KMID: 1783616
- DOI: http://doi.org/10.5051/jpis.2011.41.5.218
Abstract
- PURPOSE
Micro-computed tomography (micro-CT) has been widely used in the evaluation of regenerated bone tissue but the reliability of micro-CT has not yet been established. This study evaluated the correlation between histomorphometric analysis and micro-CT analysis in performing new bone formation measurement.
METHODS
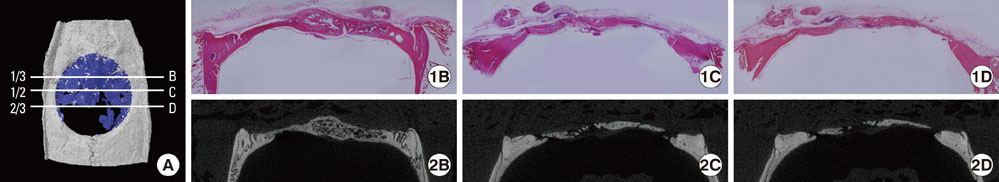
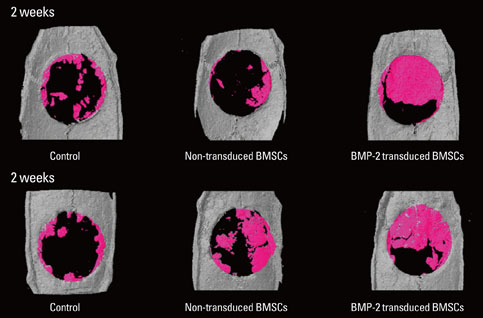
Critical-size calvarial defects were created using a 8 mm trephine bur in a total of 24 Sprague-Dawley rats, and collagen gel mixed with autogenous rat bone marrow stromal cells (BMSCs) or autogenous rat BMSCs transduced by adenovirus containing bone morphogenic protein-2 (BMP-2) genes was loaded into the defect site. In the control group, collagen gel alone was loaded into the defect. After 2 and 4 weeks, the animals were euthanized and calvaria containing defects were harvested. Micro-CT analysis and histomorphometric analysis of each sample were accomplished and the statistical evaluation about the correlation between both analyses was performed.
RESULTS
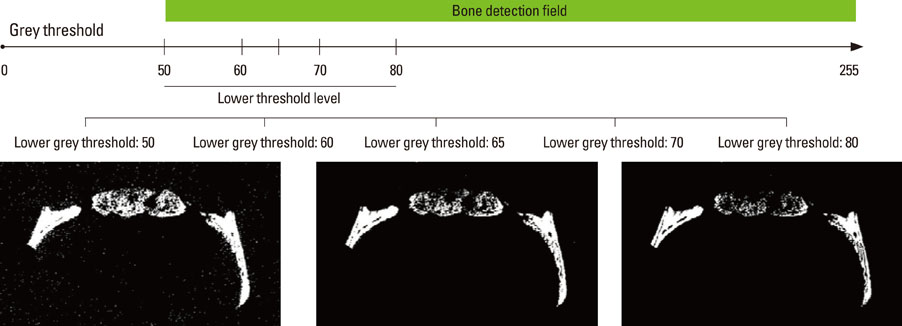
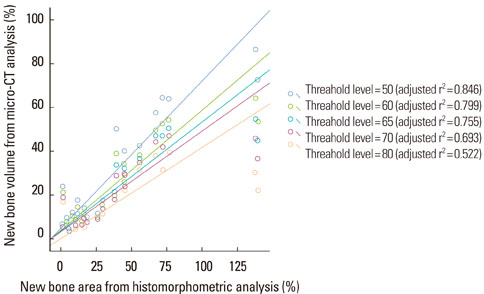
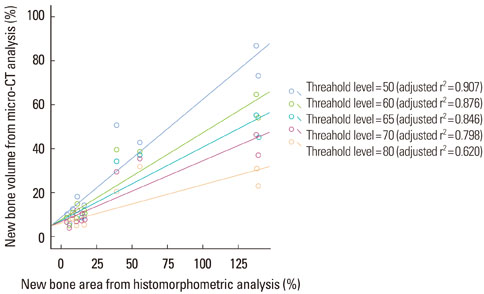
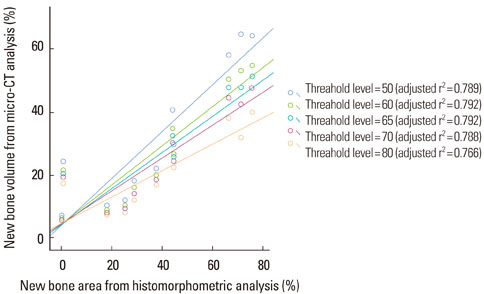
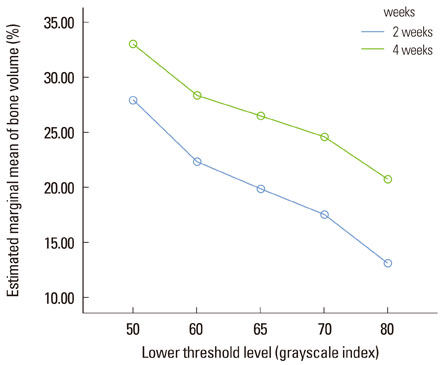
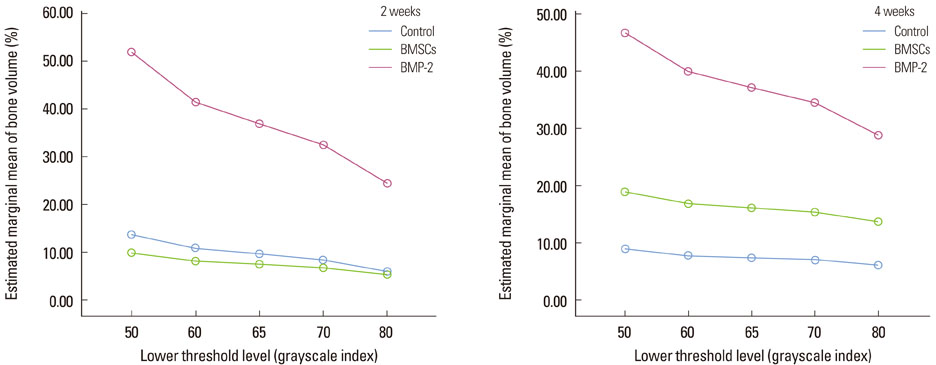
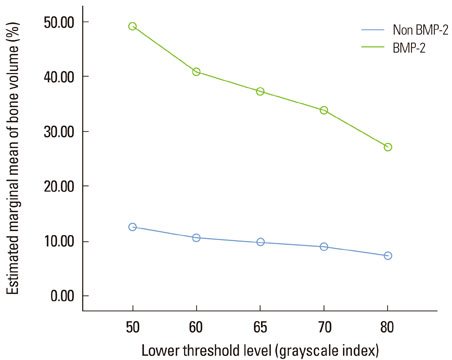
New bone formation of the BMP-2 group was greater than that of the other groups at 2 and 4 weeks in both histomorphometric analysis and micro-CT analysis (P=0.026, P=0.034). Histomorphometric analysis of representative sections showed similar results to histomorphometric analysis with a mean value of 3 sections. Measurement of new bone formation was highly correlated between histomorphometric analysis and micro-CT analysis, especially at the low lower threshold level at 2 weeks (adjusted r2=0.907, P<0.001). New bone formation of the BMP-2 group analyzed by micro-CT tended to decline sharply with an increasing lower threshold level, and it was statistically significant (P<0.001).
CONCLUSIONS
Both histomorphometric analysis and micro-CT analysis were valid methods for measurement of the new bone in rat calvarial defects and the ability to detect the new bone in micro-CT analysis was highly influenced by the threshold level in the BMP-2 group at early stage.
MeSH Terms
Figure
Reference
-
1. Ryoo HM, Lee MH, Kim YJ. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006. 366:51–57.
Article2. Seol YJ, Park YJ, Lee SC, Kim KH, Lee JY, Kim TI, et al. Enhanced osteogenic promotion around dental implants with synthetic binding motif mimicking bone morphogenetic protein (BMP)-2. J Biomed Mater Res A. 2006. 77:599–607.
Article3. Kofron MD, Laurencin CT. Bone tissue engineering by gene delivery. Adv Drug Deliv Rev. 2006. 58:555–576.
Article4. Lutz R, Park J, Felszeghy E, Wiltfang J, Nkenke E, Schlegel KA. Bone regeneration after topical BMP-2-gene delivery in circumferential peri-implant bone defects. Clin Oral Implants Res. 2008. 19:590–599.
Article5. Jiang XQ, Sun XJ, Lai HC, Zhao J, Wang SY, Zhang ZY. Maxillary sinus floor elevation using a tissue-engineered bone complex with beta-TCP and BMP-2 gene-modified bMSCs in rabbits. Clin Oral Implants Res. 2009. 20:1333–1340.
Article6. Evans C. Gene therapy for the regeneration of bone. Injury. 2011. 42:599–604.
Article7. Fischer J, Kolk A, Wolfart S, Pautke C, Warnke PH, Plank C, et al. Future of local bone regeneration - Protein versus gene therapy. J Craniomaxillofac Surg. 2011. 39:54–64.
Article8. Koh JT, Zhao Z, Wang Z, Lewis IS, Krebsbach PH, Franceschi RT. Combinatorial gene therapy with BMP2/7 enhances cranial bone regeneration. J Dent Res. 2008. 87:845–849.
Article9. Patterson J, Siew R, Herring SW, Lin AS, Guldberg R, Stayton PS. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials. 2010. 31:6772–6781.
Article10. Huang YC, Simmons C, Kaigler D, Rice KG, Mooney DJ. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4). Gene Ther. 2005. 12:418–426.
Article11. Chang PC, Seol YJ, Cirelli JA, Pellegrini G, Jin Q, Franco LM, et al. PDGF-B gene therapy accelerates bone engineering and oral implant osseointegration. Gene Ther. 2010. 17:95–104.
Article12. Young S, Patel ZS, Kretlow JD, Murphy MB, Mountziaris PM, Baggett LS, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009. 15:2347–2362.
Article13. Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008. 43:931–940.
Article14. Chang SC, Chuang H, Chen YR, Yang LC, Chen JK, Mardini S, et al. Cranial repair using BMP-2 gene engineered bone marrow stromal cells. J Surg Res. 2004. 119:85–91.15. Park EJ, Kim ES, Weber HP, Wright RF, Mooney DJ. Improved bone healing by angiogenic factor-enriched platelet-rich plasma and its synergistic enhancement by bone morphogenetic protein-2. Int J Oral Maxillofac Implants. 2008. 23:818–826.16. Gielkens PF, Schortinghuis J, de Jong JR, Huysmans MC, Leeuwen MB, Raghoebar GM, et al. A comparison of micro-CT, microradiography and histomorphometry in bone research. Arch Oral Biol. 2008. 53:558–566.
Article17. Kochi G, Sato S, Fukuyama T, Morita C, Honda K, Arai Y, et al. Analysis on the guided bone augmentation in the rat calvarium using a microfocus computerized tomography analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009. 107:e42–e48.
Article18. Shin JH, Kim KH, Kim SH, Koo KT, Kim TI, Seol YJ, et al. Ex vivo bone morphogenetic protein-2 gene delivery using gingival fibroblasts promotes bone regeneration in rats. J Clin Periodontol. 2010. 37:305–311.
Article19. Maréchal M, Luyten F, Nijs J, Postnov A, Schepers E, van Steenberghe D. Histomorphometry and micro-computed tomography of bone augmentation under a titanium membrane. Clin Oral Implants Res. 2005. 16:708–714.
Article20. Umoh JU, Sampaio AV, Welch I, Pitelka V, Goldberg HA, Underhill TM, et al. In vivo micro-CT analysis of bone remodeling in a rat calvarial defect model. Phys Med Biol. 2009. 54:2147–2161.
Article21. Kochi G, Sato S, Ebihara H, Hirano J, Arai Y, Ito K. A comparative study of microfocus CT and histomorphometry in the evaluation of bone augmentation in rat calvarium. J Oral Sci. 2010. 52:203–211.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone regeneration effects of human allogenous bone substitutes: a preliminary study
- The Application of L-Serine-Incorporated Gelatin Sponge into the Calvarial Defect of the Ovariectomized Rats
- Factors Influencing Regeneration of Calvarial Defects in Rats
- Determination of the critical diabetes duration in a streptozotocin-induced diabetic rat calvarial defect model for experimentation regarding bone regeneration
- Guided Bone Regeneration Of Calvarial Bone Defects Using Bioabsorbable Membrane And Demineralized Freeze Dried Bone In Rats