J Korean Med Sci.
2009 Dec;24(6):1078-1082. 10.3346/jkms.2009.24.6.1078.
Long-term Effectiveness and Tolerability of Topiramate in Children with Epilepsy under the Age of 2 Years: 4-Year Follow-up
- Affiliations
-
- 1Department of Pediatrics, School of Medicine, Kyungpook National University, Daegu, Korea. shkwon@knu.ac.kr
- 2Department of Neurology, School of Medicine, Kyungpook National University, Daegu, Korea.
- KMID: 1783136
- DOI: http://doi.org/10.3346/jkms.2009.24.6.1078
Abstract
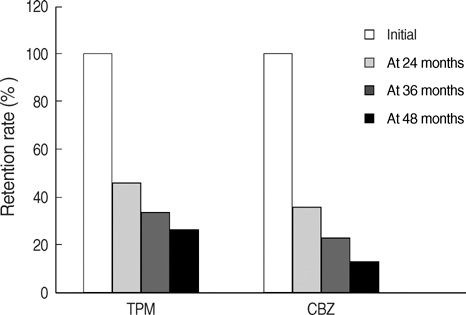
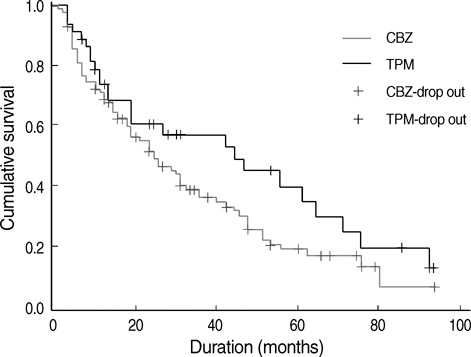
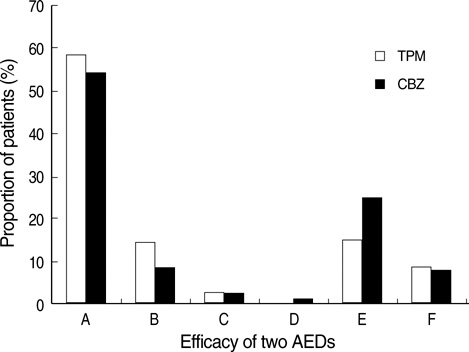
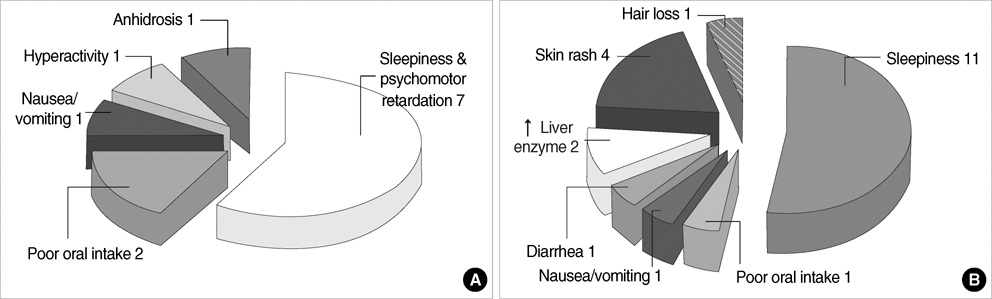
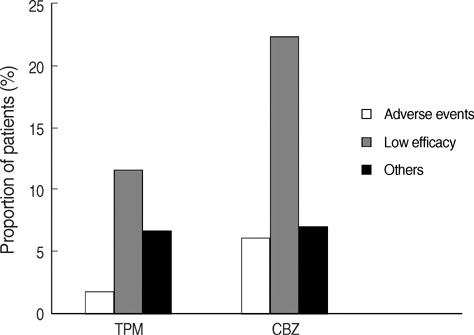
- This is a long-term, open label, observational study aimed to broaden our clinical experiences in managing infants and toddlers with epilepsy. The long-term retention rate and side effects of topiramate (TPM) in them were evaluated and compared with carbamazepine (CBZ). A total of 146 children were involved in the study (TPM=41, CBZ=105). The retention rates at 24 , 36, and 48 months were 46.3%, 34.1%, 26.8% for TPM and 36.2%, 23.8%, 13.3% for CBZ, respectively. At 6 months after starting antiepileptic drugs (AED), the seizure freedom or clinical efficacy (seizure reduction rate more than 50 percent) were 73.2% for TPM and 62.9% for CBZ. The major side effects led to discontinuation included psychomotor slowing, poor oral intake from TPM and sleepiness and skin rash from CBZ. TPM was discontinued due to side effects in one case (2.4%) and lack of efficacy in five cases (12.2%), whereas CBZ was discontinued due to lack of efficacy (22.9%) and side effects (6.7%). As compared with CBZ, TPM showed the same long-term retention rate in children under the age of 2 yr, and no serious side effects. It is therefore concluded that TPM can be considered as a major AED for treating children with epilepsy under the age of 2 yr.
Keyword
MeSH Terms
Figure
Reference
-
1. Aicardi J. Risks and benefits of new antiepileptic agents in children. Rev Neurol. 2000. 31:376–381.2. Bourgeois BF. Pharmacokinetics and pharmacodynamics of topiramate. J Child Neurol. 2000. 15:Suppl 1. S27–S30.
Article3. Bebin M. Pediatric partial and generalized seizures. J Child Neurol. 2002. 17:Suppl 1. S65–S69.
Article4. Blumkin L, Lerman-Sagie T, Houri T, Gilad E, Nissenkorn A, Ginsberg M, Watemberg N. Pediatric refractory partial status epilepticus responsive to topiramate. J Child Neurol. 2005. 20:239–241.
Article5. Bootsma HP, Aldenkamp AP, Diepman L, Hulsman J, Lambrechts D, Leenen L, Majoie M, Schellekens A, de Krom M. The effect of antiepileptic drugs on cognition: patient perceived cognitive problems of topiramate versus levetiracetam in clinical practice. Epilepsia. 2006. 47:Suppl 2. 24–27.
Article6. Wheless JW. Use of topiramate in childhood generalized seizure disorders. J Child Neurol. 2000. 15:Suppl 1. S7–13.
Article7. Waugh J, Goa KL. Topiramate: as monotherapy in newly diagnosed epilepsy. CNS Drugs. 2003. 17:985–992.8. Markind JE. Topiramate: a new antiepileptic drug. Am J Health Syst Pharm. 1998. 55:554–562.
Article9. Ormrod D, McClellan K. Topiramate: a review of its use in childhood epilepsy. Paediatr Drugs. 2001. 3:293–319.10. Nicolson A, Appleton RE, Chadwick DW, Smith DF. The relationship between treatment with valproate, lamotrigine, and topiramate and the prognosis of the idiopathic generalised epilepsies. J Neurol Neurosurg Psychiatry. 2004. 75:75–79.11. Privitera MD, Brodie MJ, Mattson RH, Chadwick DW, Neto W, Wang S. Topiramate, carbamazepine and valproate monotherapy: double-blind comparison in newly diagnosed epilepsy. Acta Neurol Scand. 2003. 107:165–175.
Article12. Korinthenberg R, Schreiner A. Topiramate in children with west syndrome: a retrospective multicenter evaluation of 100 patients. J Child Neurol. 2007. 22:302–306.
Article13. Rosenfeld WE, Doose DR, Walker SA, Baldassarre JS, Reife RA. A study of topiramate pharmacokinetics and tolerability in children with epilepsy. Pediatr Neurol. 1999. 20:339–344.
Article14. Mikaeloff Y, de Saint-Martin A, Mancini J, Peudenier S, Pedespan JM, Vallee L, Motte J, Bourgeois M, Arzimanoglou A, Dulac O, Chiron C. Topiramate: efficacy and tolerability in children according to epilepsy syndromes. Epilepsy Res. 2003. 53:225–232.
Article15. Mikaeloff Y, Rey E, Soufflet C, d'Athis P, Echenne B, Vallee L, Bouhours P, Grinspan A, Dulac O, Pons G, Chiron C. Topiramate pharmacokinetics in children with epilepsy aged from 6 months to 4 years. Epilepsia. 2004. 45:1448–1452.
Article16. Malphrus AD, Wilfong AA. Use of the newer antiepileptic drugs in pediatric epilepsies. Curr Treat Options Neurol. 2007. 9:256–267.
Article17. Chung S, Wang N, Hank N. Comparative retention rates and long-term tolerability of new antiepileptic drugs. Seizure. 2007. 16:296–304.
Article18. Zaccara G, Messori A, Cincotta M, Burchini G. Comparison of the efficacy and tolerability of new antiepileptic drugs: what can we learn from long-term studies? Acta Neurol Scand. 2006. 114:157–168.
Article19. Bootsma HP, Coolen F, Aldenkamp AP, Arends J, Diepman L, Hulsman J, Lambrechts D, Leenen L, Majoie M, Schellekens A, de Krom M. Topiramate in clinical practice: long-term experience in patients with refractory epilepsy referred to a tertiary epilepsy center. Epilepsy Behav. 2004. 5:380–387.
Article20. Moreland EC, Griesemer DA, Holden KR. Topiramate for intractable childhood epilepsy. Seizure. 1999. 8:38–40.
Article21. Mohamed K, Appleton R, Rosenbloom L. Efficacy and tolerability of topiramate in childhood and adolescent epilepsy: a clinical experience. Seizure. 2000. 9:137–141.
Article22. Voronkova KV, Pylaeva OA, Petrukhin AS. Efficacy of topiramate (Topamax) in epileptic patients of different ages. Neurosci Behav Physiol. 2007. 37:547–551.
Article23. Nieto-Barrera M, Candau R, Nieto-Jimenez M, Correa A, del Portal LR. Topiramate in the treatment of severe myoclonic epilepsy in infancy. Seizure. 2000. 9:590–594.
Article24. Zou LP, Lin Q, Qin J, Cai FC, Liu ZS, Mix E. Evaluation of open-label topiramate as primary or adjunctive therapy in infantile spasms. Clin Neuropharmacol. 2008. 31:86–92.
Article25. Nieto-Barrera M, Nieto-Jimenez M, Candau R, Ruiz del Portal L. Anhidrosis and hyperthermia associated with treatment with topiramate. Rev Neurol. 2002. 34:114–116.26. Mukhin K, Glukhova L, Petrukhin AS, Mironov MB, Sobornova AM. Topamax in monotherapy of epilepsy. Zh Nevrol Psikhiatr Im S S Korsakova. 2004. 104:35–40.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Safety and Efficacy of Topiramate Monotherapy in Children with Recent-onset Seizures
- The Effectiveness and Tolerability of Topiramate and Lidocaine Add-on Therapy in Children with Refractory Status Epilepticus
- Change of Body Weight According to Antiepileptic Drugs in Children with Epilepsy:Valproic Acid vs. Topiramate
- Ketogenic Diet for Intractable Epilepsy in Infancy and Childhood: Anti-epileptic Efficacy and Tolerability
- Cognitive and Behavioral Problems and the Effectiveness of Topiramate Once per Day in the Control of Benign Childhood Epilepsy with Centrotemporal Spikes