J Korean Med Sci.
2008 Oct;23(5):857-863. 10.3346/jkms.2008.23.5.857.
The Effect of Interleukin-4 and Amphiregulin on the Proliferation of Human Airway Smooth Muscle Cells and Cytokine Release
- Affiliations
-
- 1Department of Pediatrics, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. jy7.shim@samsung.com
- KMID: 1783073
- DOI: http://doi.org/10.3346/jkms.2008.23.5.857
Abstract
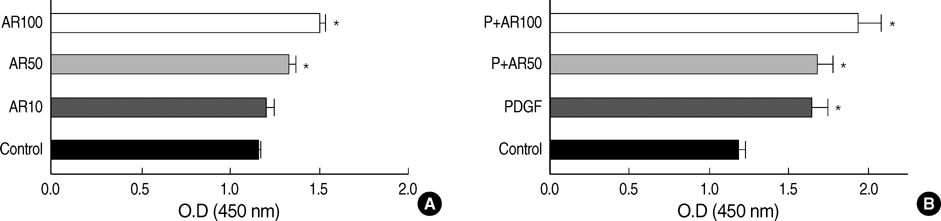
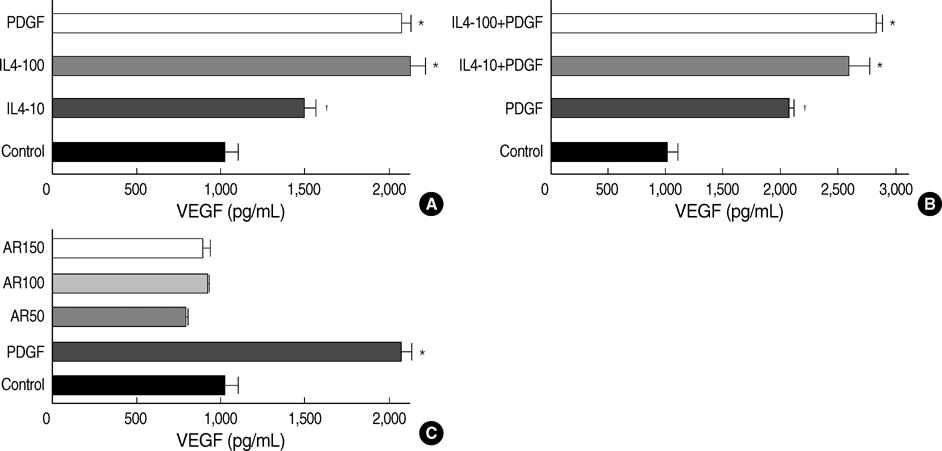
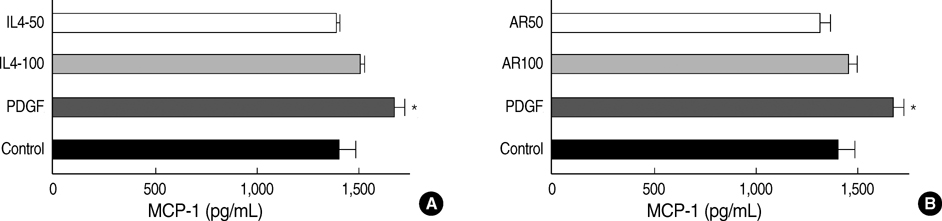
- Airway smooth muscle (ASM) hyperplasia and angiogenesis are important features associated with airway remodeling. We investigated the effect of IL-4 and amphiregulin, an epidermal growth factor family member, on the proliferation of human ASM cells and on the release of vascular endothelial growth factor (VEGF) and monocyte chemotactic protein (MCP)-1 from human ASM cells. Human ASM cells were growth-arrested for 48 hr and incubated with platelet-derived growth factor (PDGF)- BB, interleukin (IL)-4, amphiregulin, and VEGF to evaluate cell proliferation. The cells were treated with PDGF, IL-4 and amphiregulin to evaluate the release of VEGF, MCP-1. IL-4 suppressed unstimulated and PDGF-stimulated ASM cell proliferation. Amphiregulin stimulated ASM cell proliferation in a dose-dependent manner. VEGF did not have any influence on ASM cell proliferation. IL-4 stimulated VEGF secretion by the ASM cells in a dose-dependent manner and showed added stimulatory effects when co-incubated with PDGF. Amphiregulin did not promote VEGF secretion. IL-4 and amphiregulin showed no stimulatory effects on MCP-1 secretion. The results of this study showed that IL-4 had bifunctional effects on airway remodeling, one was the suppression of the proliferation of the ASM cells and the other was the promotion of VEGF release by the ASM cells, and amphiregulin can promote human ASM cell proliferation.
Keyword
MeSH Terms
-
Bronchi/metabolism
Cell Proliferation
Cells, Cultured
Chemokine CCL2/metabolism
Chemokine CCL3/metabolism
Cytokines/metabolism
*Gene Expression Regulation
Glycoproteins/*physiology
Humans
Intercellular Signaling Peptides and Proteins/*physiology
Interleukin-4/metabolism/*physiology
Models, Biological
Myocytes, Smooth Muscle/*metabolism
Vascular Endothelial Growth Factor A/metabolism
Figure
Reference
-
1. Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003. 111:291–297.
Article2. Lazaar AL, Panettieri RA Jr. Airway smooth muscle: a modulator of airway remodeling in asthma. J Allergy Clin Immunol. 2005. 116:488–495.
Article3. Richter A, Puddicombe SM, Lordan JL, Bucchieri F, Wilson SJ, Djukanovic R, Dent G, Holgate ST, Davies DE. The contribution of interleukin (IL)-4 and IL-13 to the epithelial-mesenchymal trophic unit in asthma. Am J Respir Cell Mol Biol. 2001. 25:385–391.
Article4. Hirst SJ, Hallsworth MP, Peng Q, Lee TH. Selective induction of eotaxin release by interleukin-13 or interleukin-4 in human airway smooth muscle cells is synergistic with interleukin-1beta and is mediated by the interleukin-4 receptor alpha -chain. Am J Respir Crit Care Med. 2002. 165:1161–1171.5. Kelly-Welch AE, Melo ME, Smith E, Ford AQ, Haudenschild C, Noben-Trauth N, Keegan AD. Complex role of the IL-4 receptor alpha in a murine model of airway inflammation: expression of the IL-4 receptor alpha on nonlymphoid cells of bone marrow origin contributes to severity of inflammation. J Immunol. 2004. 172:4545–4555.6. Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med. 2004. 10:493–499.
Article7. Rankin JA, Picarella DE, Geba GP, Temann UA, Prasad B, DiCosmo B, Tarallo A, Stripp B, Whitsett J, Flavell RA. Phenotypic and physiologic characterization of transgenic mice expressing interleukin 4 in the lung: lymphocytic and eosinophilic inflammation without airway hyperreactivity. Proc Natl Acad Sci USA. 1996. 93:7821–7825.
Article8. Parameswaran K, Radford K, Fanat A, Stephen J, Bonnans C, Levy BD, Janssen LJ, Cox PG. Modulation of human airway smooth muscle migration by lipid mediators and Th-2 cytokines. Am J Respir Cell Mol Biol. 2007. 37:240–247.
Article9. Monroe JG, Haldar S, Prystowsky MB, Lammie P. Lymphokine regulation of inflammatory processes: interleukin-4 stimulates fibroblast proliferation. Clin Immunol Immunopathol. 1988. 49:292–298.
Article10. Vadiveloo PK, Stanton HR, Cochran FW, Hamilton JA. Interleukin-4 inhibits human smooth muscle cell proliferation. Artery. 1994. 21:161–181.11. Toi M, Harris AL, Bicknell R. Interleukin-4 is a potent mitogen for capillary endothelium. Biochem Biophys Res Commun. 1991. 174:1287–1293.
Article12. Hawker KM, Johnson PR, Hughes JM, Black JL. Interleukin-4 inhibits mitogen-induced proliferation of human airway smooth muscle cells in culture. Am J Physiol. 1998. 275:L469–L477.13. Estes ML, Iwasaki K, Jacobs BS, Barna BP. Interleukin-4 downregulates adult human astrocyte DNA synthesis and proliferation. Am J Pathol. 1993. 143:337–341.14. Lee IY, Kim J, Ko EM, Jeoung EJ, Kwon YG, Choe J. Interleukin-4 inhibits the vascular endothelial growth factor- and basic fibroblast growth factor-induced angiogenesis in vitro. Mol Cells. 2002. 14:115–121.15. Feltis BN, Wignarajah D, Zheng L, Ward C, Reid D, Harding R, Walters EH. Increased vascular endothelial growth factor and receptors: Relationship to angiogenesis in asthma. Am J Respir Crit Care Med. 2006. 173:1201–1207.16. Wen FQ, Liu X, Manda W, Terasaki Y, Kobayashi T, Abe S, Fang Q, Ertl R, Manouilova L, Rennard SI. Th2 cytokine-enhanced and TGF-beta-enhanced vascular endothelial growth factor production by cultured human airway smooth muscle cells is attenuated by IFN-gamma and corticosteroids. J Allergy Clin Immunol. 2003. 111:1307–1318.17. Faffe DS, Flynt L, Bourgeois K, Panettieri RA Jr, Shore SA. Interleukin-13 and interleukin-4 induce vascular endothelial growth factor release from airway smooth muscle cells: role of vascular endothelial growth factor genotype. Am J Respir Cell Mol Biol. 2006. 34:213–218.18. Hong KH, Cho ML, Min SY, Shin YJ, Yoo SA, Choi JJ, Kim WU, Song SW, Cho CS. Effect of interleukin-4 on vascular endothelial growth factor production in rheumatoid synovial fibroblasts. Clin Exp Immunol. 2007. 147:573–579.
Article19. Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004. 20:267–277.
Article20. Leigh R, Ellis R, Wattie JN, Hirota JA, Matthaei KI, Foster PS, O'Byrne PM, Inman MD. Type 2 cytokines in the pathogenesis of sustained airway dysfunction and airway remodeling in mice. Am J Respir Crit Care Med. 2004. 169:860–867.
Article21. Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999. 103:779–788.
Article22. Ma B, Blackburn MR, Lee CG, Homer RJ, Liu W, Flavell RA, Boyden L, Lifton RP, Sun CX, Young HW, Elias JA. Adenosine metabolism and murine strain-specific IL-4-induced inflammation, emphysema, and fibrosis. J Clin Invest. 2006. 116:1274–1283.
Article23. Bassa BV, Noh JW, Ganji SH, Shin MK, Roh DD, Kamanna VS. Lysophosphatidylcholine stimulates EGF receptor activation and mesangial cell proliferation: regulatory role of Src and PKC. Biochim Biophys Acta. 2007. 1771:1364–1371.
Article24. Toi M, Bicknell R, Harris AL. Inhibition of colon and breast carcinoma cell growth by interleukin-4. Cancer Res. 1992. 52:275–279.25. Kim J, Cheon IS, Won YJ, Na HJ, Kim YM, Choe J. IL-4 inhibits cell cycle progression of human umbilical vein endothelial cells by affecting p53, p21(Waf1), cyclin D1, and cyclin E expression. Mol Cells. 2003. 16:92–96.26. Barna BP, Estes ML, Pettay J, Iwasaki K, Zhou P, Barnett GH. Human astrocyte growth regulation. Interleukin-4 sensitivity and receptor expression. J Neuroimmunol. 1995. 60:75–81.
Article27. Saito A, Okazaki H, Sugawara I, Yamamoto K, Takizawa H. Potential action of IL-4 and IL-13 as fibrogenic factors on lung fibroblasts in vitro. Int Arch Allergy Immunol. 2003. 132:168–176.
Article28. Hua K, Deng J, Harp JB. Interleukin-4 inhibits platelet-derived growth factor-induced preadipocyte proliferation. Cytokine. 2004. 25:61–67.
Article29. Laporte JC, Moore PE, Baraldo S, Jouvin MH, Church TL, Schwartzman IN, Panettieri RA Jr, Kinet JP, Shore SA. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med. 2001. 164:141–148.
Article30. Orsini MJ, Krymskaya VP, Eszterhas AJ, Benovic JL, Panettieri RA Jr, Penn RB. MAPK superfamily activation in human airway smooth muscle: mitogenesis requires prolonged p42/p44 activation. Am J Physiol. 1999. 277:L479–L488.31. Kriebel P, Patel BK, Nelson SA, Grusby MJ, LaRochelle WJ. Consequences of STAT6 deletion on Sis/PDGF- and IL-4-induced proliferation and transcriptional activation in murine fibroblasts. Oncogene. 1999. 18:7294–7302.
Article32. Bosse Y, Stankova J, Rola-Pleszezynski M. The Th2 cytokines, IL-4 and IL-13, induce human bronchial smooth muscle proliferation. J Allergy Clin Immunol (abstract book). 2006. 117:S721.33. Yu J, Moon A, Kim HR. Both platelet-derived growth factor receptor (PDGFR)-alpha and PDGFR-beta promote murine fibroblast cell migration. Biochem Biophys Res Commun. 2001. 282:697–700.34. Hirst SJ, Barnes PJ, Twort CH. PDGF isoform-induced proliferation and receptor expression in human cultured airway smooth muscle cells. Am J Physiol. 1996. 270:L415–L428.
Article35. Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997. 156:229–233.
Article36. Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol. 2001. 107:1034–1038.
Article37. Kato M, Inazu T, Kawai Y, Masamura K, Yoshida M, Tanaka N, Miyamoto K, Miyamori I. Amphiregulin is a potent mitogen for the vascular smooth muscle cell line, A7r5. Biochem Biophys Res Commun. 2003. 301:1109–1115.
Article38. Falk A, Frisen J. Amphiregulin is a mitogen for adult neural stem cells. J Neurosci Res. 2002. 69:757–762.
Article39. Hurbin A, Dubrez L, Coll JL, Favrot MC. Inhibition of apoptosis by amphiregulin via an insulin-like growth factor-1 receptor-dependent pathway in non-small cell lung cancer cell lines. J Biol Chem. 2002. 277:49127–49133.
Article40. Okumura S, Sagara H, Fukuda T, Saito H, Okayama Y. Fcepsilon-RI-mediated amphiregulin production by human mast cells increases mucin gene expression in epithelial cells. J Allergy Clin Immunol. 2005. 115:272–279.41. Wang SW, Oh CK, Cho SH, Hu G, Martin R, Demissie-Sanders S, Li K, Moyle M, Yao Z. Amphiregulin expression in human mast cells and its effect on the primary human lung fibroblasts. J Allergy Clin Immunol. 2005. 115:287–294.
Article42. Kumar RK, Herbert C, Foster PS. Expression of growth factors by airway epithelial cells in a model of chronic asthma: regulation and relationship to subepithelial fibrosis. Clin Exp Allergy. 2004. 34:567–575.
Article43. Kazi AS, Lotfi S, Goncharova EA, Tliba O, Amrani Y, Krymskaya VP, Lazaar AL. Vascular endothelial growth factor-induced secretion of fibronectin is ERK dependent. Am J Physiol Lung Cell Mol Physiol. 2004. 286:L539–L545.
Article44. Pype JL, Dupont LJ, Menten P, Van Coillie E, Opdenakker G, Van Damme J, Chung KF, Demedts MG, Verleden GM. Expression of monocyte chemotactic protein (MCP)-1, MCP-2, and MCP-3 by human airway smooth-muscle cells. Modulation by corticosteroids and T-helper 2 cytokines. Am J Respir Cell Mol Biol. 1999. 21:528–536.45. Hayes IM, Jordan NJ, Towers S, Smith G, Paterson JR, Earnshaw JJ, Roach AG, Westwick J, Williams RJ. Human vascular smooth muscle cells express receptors for CC chemokines. Arterioscler Thromb Vasc Biol. 1998. 18:397–403.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Adiponectin Receptors and the Effects of Adiponectin and Leptin on Airway Smooth Muscle Cells
- Dexamethasone Attenuates PDGF- and TGF-beta-enhanced Vascular Endothelial Growth Factor Production in Cultured Human Bronchial Smooth Muscle Cells
- Transforming growth factor-beta promoted vascular endothelial growth factor release by human lung fibroblasts
- IL-13 R110Q, a Naturally Occurring IL-13 Polymorphism, Confers Enhanced Functional Activity in Cultured Human Bronchial Smooth Muscle Cells
- Airway Remodelling in Asthma